The Mechanism of Action of REAGILA
- The mechanism of action of cariprazine is not fully known1.
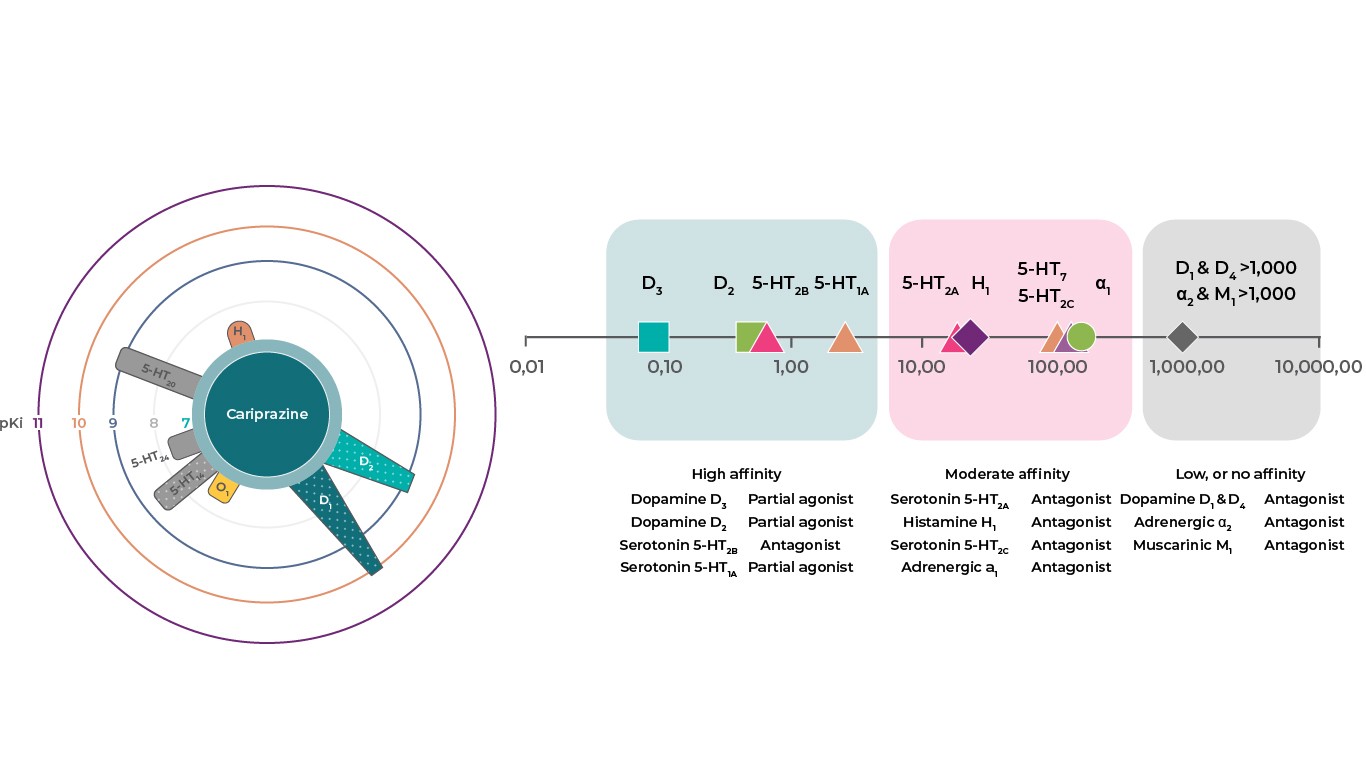
- The therapeutic effect of cariprazine may be mediated through a combination of partial agonist activity at dopamine D3, D2 and serotonin 5‑HT1A receptors and antagonist activity at serotonin 5‑HT2B receptors1.
- Cariprazine has low affinity for serotonin 5‑HT2C and adrenergic α1 receptors, with no appreciable affinity for cholinergic muscarinic receptors1.
- Cariprazine has 2 major active metabolites, desmethyl‑cariprazine and didesmethyl‑cariprazine, which have a similar in vitro receptor binding and functional activity profile as the parent drug1.
In this section
The mechanism of action for all antipsychotics, including cariprazine, is not fully known1. However, based on its receptor profile and numerous studies including in vivo preclinical studies and human PET studies, enough evidence exists to elucidate the key factors contributing to the mechanism of action of cariprazine.
Receptor Profile of Cariprazine
Cariprazine has high affinity for dopamine D3 and D2 receptors as well as serotonin 5‑HT2B and 5‑HT1A receptors, moderate affinity to 5‑HT2A, histamine H1, and sigma σ1 receptors, low affinity for serotonin 5‑HT2C and adrenergic α 1 receptors, and no appreciable affinity for cholinergic muscarinic receptors1,2. It is believed that cariprazine’s therapeutic effects are mediated through some combination of partial agonist activity at dopamine D3, D2, and serotonin 5‑HT1A receptors and antagonist activity at 5‑HT2B receptors1.
Reference: Adapted from Kiss. J Pharmacol Exp Ther 2010.333:328–340; Partial agonism is represented with dots, circles show pKi values.2
Antipsychotics are generally thought to be effective against positive symptoms of schizophrenia, though they are less effective for treating cognitive deficits and negative symptoms associated with schizophrenia. Results from animal models suggest that the dopamine D3 receptor plays an important role in regulating cognition, mood, and social functions3. Most currently available antipsychotics have a lower affinity for D3 receptors relative to the very high affinity of dopamine itself for the D3 receptor that is why they do not occupy dopamine D3 receptors in the living brain3. Cariprazine, on the other hand, is unique among antipsychotics in that it has a higher potency for the D3 receptor than dopamine itself, meaning that the net effect of cariprazine administration is D3 receptor blockade4-8. D3 receptor blockade in patients with schizophrenia is hypothesized to result in procognitive, antidepressant, and reduced negative symptom effects3. Cariprazine has demonstrated efficacy in behavioral preclinical models of negative/mood symptoms and cognitive impairment9–14. The efficacy in these animal models has been shown to be at least partially dependent on its activity at the dopamine D3 receptor, since its effects on anhedonia and cognitive impairment were apparent in wild-type mice but not in D3 receptor‑knockout mice10,14.
Receptor Occupancy with Cariprazine
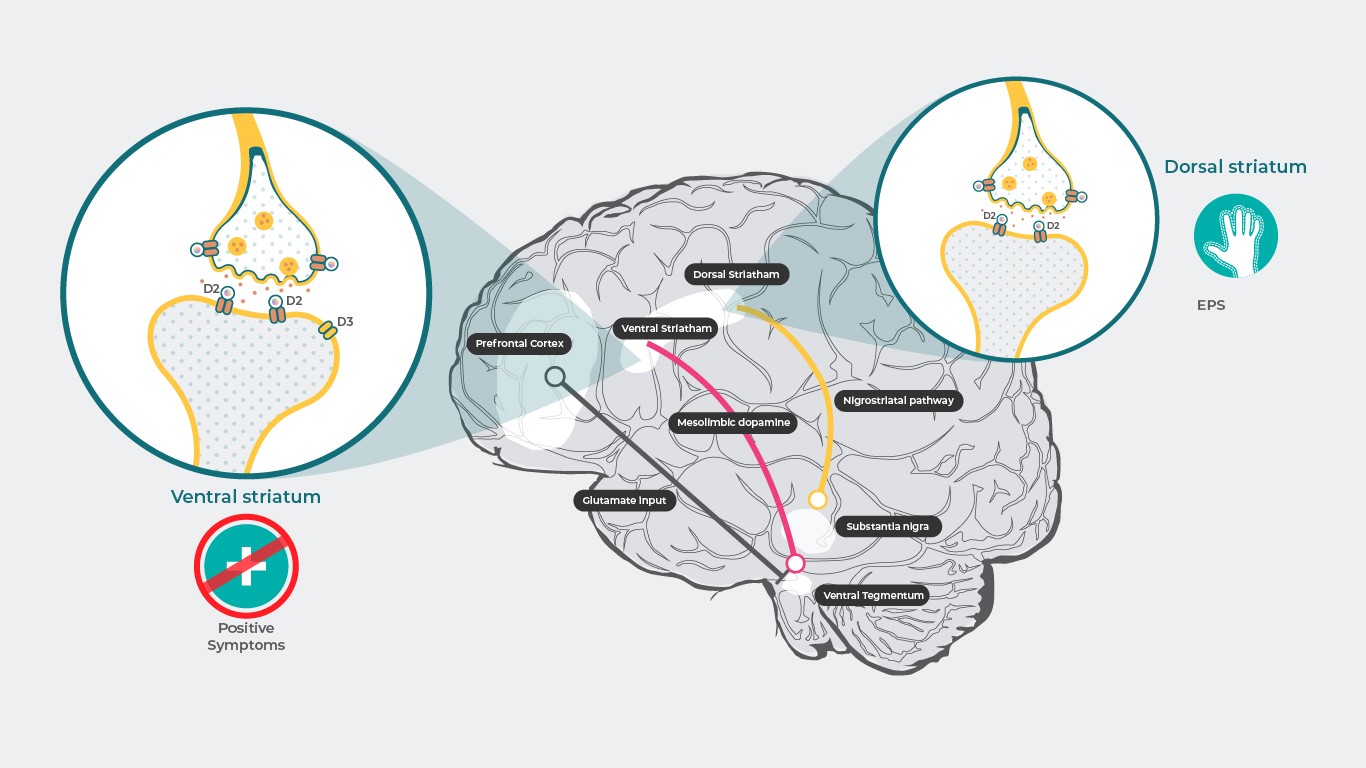
At pharmacologically effective doses, cariprazine shows relatively similar occupancy at both D3 and D2 receptors, as demonstrated by in vivo nonclinical and human PET studies15. Dose-dependent occupancy of dopamine D3 and D2 receptors was observed in patients with schizophrenia dosed with cariprazine in the therapeutic dose range1. In brain regions with higher D3 receptor expression, cariprazine displayed greater preferential occupancy for D3 versus D2 receptors15. Drug affinities for D3 receptors for many agents are about the same than their affinities for D2 receptors, but they are mostly lower than dopamine’s affinity for the D3 receptor3. Therefore, effects attributed to D3 receptor blockade are not associated with any drug other than cariprazine, since in the living brain, in the presence of natural dopamine, D3 receptors are not blocked by any antipsychotic other than cariprazine3,4. Concerning safety, blockade of the nigrostriatal or tuberoinfundibular pathways, which is associated with extrapyramidal symptoms (EPS) and elevated prolactin levels, could potentially be avoided with partial agonist16; so theoretically, cariprazine should cause fewer side effects of this nature. However, as observed in clinical studies, this has proven to be true for prolactin levels, which do not increase in response to treatment with cariprazine17, although increased incidence of EPS/akathisia is noted17, for which there is no apparent explanation16.
References: Adapted from Stahl SM. CNS Spectrums (2017), 22, 375–384.3 ; Howes J Psychopharmacol. 2015 February; 29(2): 97–115.18
In vitro, cariprazine exhibits partial agonist activity at D3 receptors1. Since most D3 receptors are localized in brain areas where hyperdopaminergia is present in schizophrenia (ie, in the mesolimbic dopaminergic system)15,18, cariprazine acts as a functional antagonist on these receptors. D3 receptor antagonism is associated with pro-cognitive, antidepressant, and anti-negative symptom effects3. Confusingly, these same effects are also linked to cortical functions, where hypodopaminergia is present in schizophrenia18. Herein lies the question of how does cariprazine normalize these hypodopaminergic states by D3 receptor blockade?
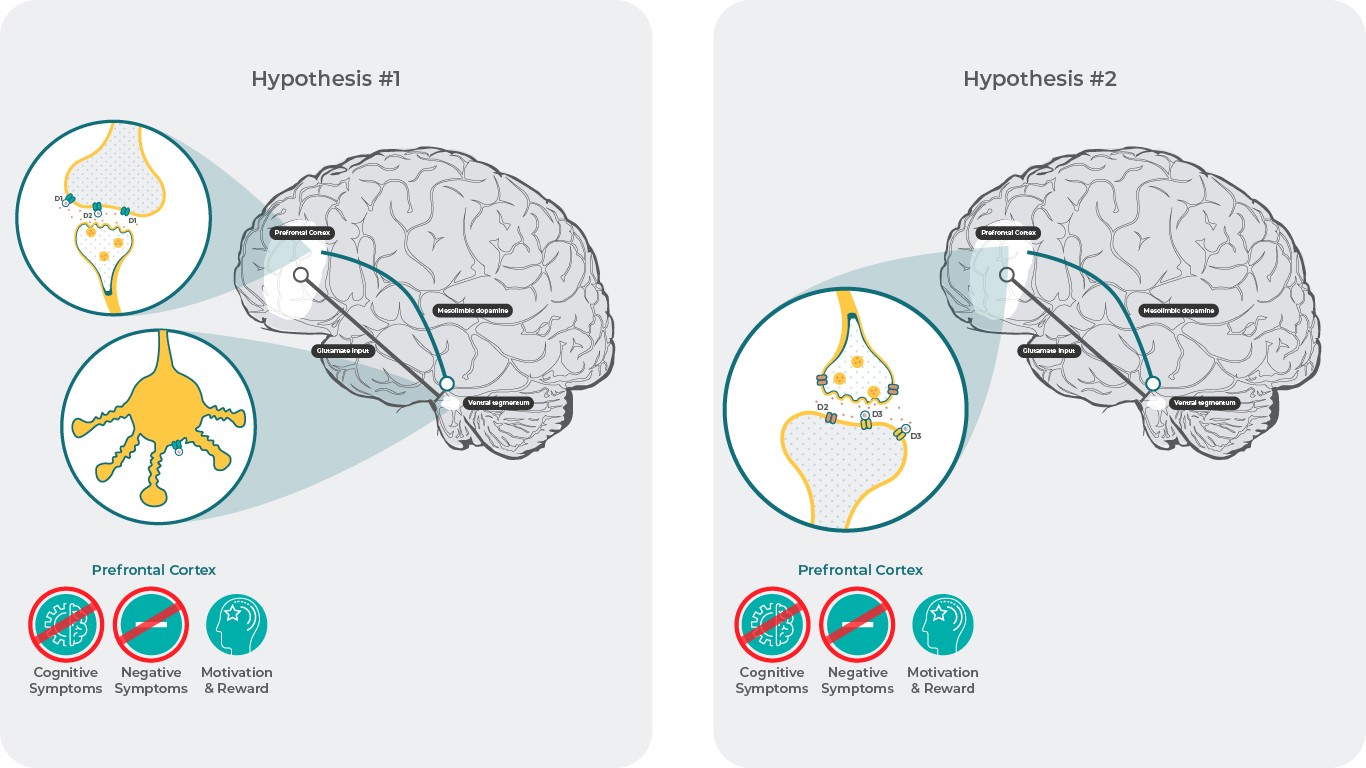
Hypothesis 1: References: Adapted from Howes J Psychopharmacol. 2015 February; 29(2): 97–115.18; Stahl SM. CNS Spectrums (2017), 22, 375–384.3
Hypothesis 2: References: Adapted from Bouthenet et al. Brain research 1991.564, 203-21928; Gurevich, Neuropsychopharmacology 1999. 20, 60-80.29; Loiseau, Eur Neuropsychopharmacol 2009.19, 23-33.30; Watson, D.J. Neuropsychopharmacology 2012.37, 770-786.31; Clarkson, J Neurosci 2017.37, 5846-5860.32; Yang, S. Cell Rep 2016.16, 1518-1526.33; Neill, J.C. Eur Neuropsychopharmacol 2016. 26, 3-14.11; Zimnisky, R. Psychopharmacology (Berl) 2013.226, 91-100.14
Hypothesis #1: Increased Neurotransmission From VTA to PFC
Negative symptoms and cognitive impairment have been associated with a hypodopaminergic state in the prefrontal cortex (PFC)18. The cause of this may be decreased dopamine release in the PFC due to activation of presynaptic dopamine D3 autoreceptors in the ventral tegmental area (VTA) that project to the PFC3. Under these conditions, it is believed that D3 receptor antagonists/partial agonists may block the inhibition of these neurons, which in turn results in an increase in dopamine levels in the PFC, normalizing the hypodopaminergic condition3. This increase in dopamine may reverse the hypodopaminergic state and result in improvements in negative symptoms and cognition via activation of D1 receptors in the PFC, which are not active in low dopamine conditions due to the low affinity of dopamine for these receptors3.
Although the exact mechanism of action of cariprazine and in fact all other antipsychotics is still unknown, results from preclinical experiments with cariprazine do not appear to support this hypothesis19-26. However, it is possible that a different effect would be seen in humans, as the mechanisms and functional consequences of somatodendritic dopamine transmission in the VTA has been reported to vary among species. These differences would be expected in vivo to affect the downstream activity of the mesocorticolimbic dopamine system and subsequent terminal release27.
Hypothesis #2: Postsynaptic D3 Receptor Binding in the PFC
Despite the relatively low expression of D3 receptor in the PFC28-29, evidence suggests that D3 receptors in the PFC can modulate PFC function and influence cognition5,6.
In rodents, local infusions of D3 receptor antagonists in the PFC resulted in efficacy in animal models of negative symptoms and cognition; efficacy was not seen in the nucleus accumbens (NAc) or striatum30,31, suggesting that antagonizing D3 receptors locally in the PFC has procognitive effects. This ability of D3 receptors to modulate mood and cognition despite low expression may be related to the specialized location of D3 receptors in the PFC. D3 receptors have been shown to play a distinct role in regulating excitability in layer 5 pyramidal cells in the PFC32 . A different study found that D3 receptor agonists suppress axon initial segment Cav3 channel excitability through an arrestin-dependent mechanism33. Collectively, these results suggest that cariprazine antagonism at postsynaptic D3 receptors in the PFC could contribute to efficacy in negative symptoms and cognition by modulating glutamate neuron action potentials and glutamate neurotransmission. This is further supported by the positive findings that cariprazine was able to reverse PCP induced cognitive impairment in animal behavioral models11,14
Metabolites of Cariprazine
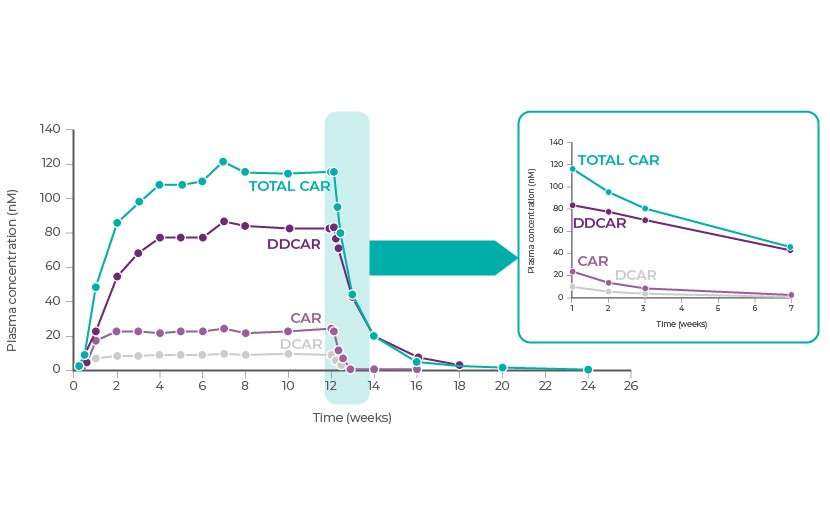
Cariprazine has 2 pharmacologically active metabolites with activities that are similar to cariprazine, desmethyl‑cariprazine (DCAR) and didesmethyl‑cariprazine (DDCAR)1. Total cariprazine (molar sum of cariprazine with DCAR and DDCAR) exposure is near 50% of steady state exposure after approximately 1 week of daily dosing, while 90% of steady state is achieved in 3 weeks. At steady state, exposure to DDCAR is approximately 2 to 3-fold higher than exposure to cariprazine, and exposure to DCAR is approximately 30% of cariprazine exposure1.
Cariprazine, DCAR, and DDCAR are primarily eliminated through hepatic metabolism (CYP3A4 and CYP2D6). The effective half-life is about 2 days for cariprazine and DCAR, 8 days for DDCAR, and ~1 week for total cariprazine. The plasma concentration of total cariprazine gradually declines after dose discontinuation, with decreases of 50% in approximately 1 week and >90% in 3 weeks1.
Reference: Adapted from Nakamura, T. et al. Clinical pharmacology study of cariprazine (MP-214) in patients with schizophrenia (12-week treatment). Drug Des. Devel. Ther. 10, 327–338 (2016)34.
References
- Reagila SmPC.
- Kiss, B. et al. Cariprazine (RGH-188), a dopamine D3 receptor-preferring, D 3/D2 dopamine receptor antagonist-partial agonist antipsychotic candidate: In vitro and neurochemical profile. J. Pharmacol. Exp. Ther. 333, 328–340 (2010).
- Stahl, S. M. Drugs for psychosis and mood: Unique actions at D3, D2, and D1 dopamine receptor subtypes. CNS Spectr. 22, 375–384 (2017).
- Stahl, S. M. Mechanism of action of cariprazine. CNS Spectr. 21, 123–127 (2016).
- Gross, G. & Drescher, K. The role of dopamine D 3 receptors in antipsychotic activity and cognitive functions. in Handbook of Experimental Pharmacology (eds. Geyer, M. A. & Gross, G.) 168–210 (2012). doi:10.1007/978-3-642-25758-2_7
- Nakajima, S. et al. The potential role of dopamine D3 receptor neurotransmission in cognition. Eur. Neuropsychopharmacol. 23, 799–813 (2013).
- Leggio, G. M., Bucolo, C., Platania, C. B. M., Salomone, S. & Drago, F. Current drug treatments targeting dopamine D3 receptor. Pharmacol. Ther. 165, 164–177 (2016).
- Ellenbroek, B. A. & Cesura, A. M. Antipsychotics and the dopamine–serotonin connection. in Topics in Medicinal Chemistry (eds. Celanire, S. & Poli, S.) 13, 1–49 (Springer, Cham, 2014).
- Barnes, S. A. et al. The Effects of Cariprazine and Aripiprazole on PCP-Induced Deficits on Attention Assessed in the 5-Choice Serial Reaction Time Task. Psychopharmacology (Berl). 235, 1403–1414 (2018).
- Duric, V. et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int. J. Neuropsychopharmacol. 20, 788–796 (2017).
- Neill, J. C. et al. Effects of cariprazine, a novel antipsychotic, on cognitive deficit and negative symptoms in a rodent model of schizophrenia symptomatology. Eur. Neuropsychopharmacol. 26, 3–14 (2016).
- Papp, M. et al. Attenuation of anhedonia by cariprazine in the chronic mild stress model of depression. Behav. Pharmacol. 25, 567–574 (2014).
- Watson, D. J. G. et al. The dopamine D 3 -preferring D 2 /D 3 dopamine receptor partial agonist, cariprazine, reverses behavioural changes in a rat neurodevelopmental model for schizophrenia. Eur. Neuropsychopharmacol. 26, 208–224 (2016).
- Zimnisky, R. et al. Cariprazine, a dopamine D3-receptor-preferring partial agonist, blocks phencyclidine-induced impairments of working memory, attention set-shifting, and recognition memory in the mouse. Psychopharmacology (Berl). 226, 91–100 (2013).
- Girgis, R. R. et al. Preferential binding to dopamine D3 over D2 receptors by cariprazine in patients with schizophrenia using PET with the D3/D2 receptor ligand [11C]-(+)-PHNO. Psychopharmacology (Berl). 233, 3503–3512 (2016).
- Lieberman, J. A. Dopamine partial agonists: A new class of antipsychotic. CNS Drugs 18, 251–267 (2004).
- Nasrallah, H. A. et al. The safety and tolerability of cariprazine in long-term treatment of schizophrenia: A post hoc pooled analysis. BMC Psychiatry (2017) doi:10.1186/s12888-017-1459-z.
- Howes, O., McCutcheon, R. & Stone, J. Glutamate and dopamine in schizophrenia: An update for the 21st century. J. Psychopharmacol. 29, 97–115 (2015).
- Kehr, J. et al. Effects of cariprazine on extracellular levels of glutamate, GABA, dopamine, noradrenaline and serotonin in the medial prefrontal cortex in the rat phencyclidine model of schizophrenia studied by microdialysis and simultaneous recordings of locomotor acti. Psychopharmacology (Berl). 235, 1593–1607 (2018).
- Gobert, A. et al. Functional correlates of dopamine D3 receptor activation in the rat in vivo and their modulation by the selective antagonist, (+)-S 14297: II. Both D2 and ‘silent’ D3 autoreceptors control synthesis and release in mesolimbic, mesocortical and nigrostriata. J. Pharmacol. Exp. Ther. 275, 899–913 (1995).
- Millan, M. J. et al. S18616, a highly potent spiroimidazoline agonist at α2-adrenoceptors: II. Influence on monoaminergic transmission, motor function, and anxiety in comparison with dexmedetomidine and clonidine. J. Pharmacol. Exp. Ther. 295, 1206–1222 (2000).
- Reavill, C. et al. Pharmacological actions of a novel, high-affinity, and selective human dopamine D 3 receptor antagonist, SB-277011-A. J. Pharmacol. Exp. Ther. 294, 1154–1165 (2000).
- Delcourte, S. et al. The novel atypical antipsychotic cariprazine demonstrates dopamine D2 receptor-dependent partial agonist actions on rat mesencephalic dopamine neuronal activity. CNS Neurosci. Ther. 24, 1129–1139 (2018).
- Etievant, A., Bétry, C., Arnt, J. & Haddjeri, N. Bifeprunox and aripiprazole suppress in vivo VTA dopaminergic neuronal activity via D2 and not D3 dopamine autoreceptor activation. Neurosci. Lett. 460, 82–86 (2009).
- Koeltzow, T. E. et al. Alterations in dopamine release but not dopamine autoreceptor function in dopamine D3 receptor mutant mice. J. Neurosci. 18, 2231–2238 (1998).
- Tepper, J. M., Sun, B. C., Martin, L. P. & Creese, L. Functional roles of dopamine D2 and D3 autoreceptors on nigrostriatal neurons analyzed by antisense knockdown in vivo. J. Neurosci. 17, 2519–2530 (1997).
- Courtney, N. A., Mamaligas, A. A. & Ford, C. P. Species differences in somatodendritic dopamine transmission determine D2-autoreceptor-mediated inhibition of ventral tegmental area neuron firing. J. Neurosci. 32, 13520–13528 (2012).
- Bouthenet, M. L. et al. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 564, 203–219 (1991).
- Gurevich, E. V. & Joyce, J. N. Distribution of dopamine D3 receptor expressing neurons in the human forebrain comparison with D2 receptor expressing neurons. Neuropsychopharmacology 20, 60–80 (1999).
- Loiseau, F. & Millan, M. J. Blockade of dopamine D3 receptors in frontal cortex, but not in sub-cortical structures, enhances social recognition in rats: Similar actions of D1 receptor agonists, but not of D2 antagonists. Eur. Neuropsychopharmacol. 19, 23–33 (2009).
- Watson, D. J. G. et al. Selective blockade of dopamine D 3 receptors enhances while D 2 receptor antagonism impairs social novelty discrimination and novel object recognition in rats: A key role for the prefrontal cortex. Neuropsychopharmacology 37, 770–786 (2012).
- Clarkson, R. L., Liptak, A. T., Gee, S. M., Sohal, V. S. & Bender, K. J. D3 receptors regulate excitability in a unique class of prefrontal pyramidal cells. J. Neurosci. 37, 5846–5860 (2017).
- Yang, S. et al. β-Arrestin-Dependent Dopaminergic Regulation of Calcium Channel Activity in the Axon Initial Segment. Cell Rep. 16, 1518–1526 (2016).
- Nakamura, T. et al. Clinical pharmacology study of cariprazine (MP-214) in patients with schizophrenia (12-week treatment). Drug Des. Devel. Ther. 10, 327–338 (2016).
CNS Spectrums
Mechanism of action of cariprazine.
REAGILA IN ACUTE SCHIZOPHRENIAOUR PRODUCT IN ACUTE SCHIZOPHRENIA
Once the diagnosis of schizophrenia is made, clinicians, patients, and families have important treatment decisions to make. Although it seems intuitive to say tOnce the diagnosis of schizophrenia is made, clinicians, patients, and families have important treatment decisions to make. Although it seems intuitive to say t
more…REAGILA AND DAILY FUNCTIONINGOUR PRODUCT AND DAILY FUNCTIONING
Negative symptom improvement must be accompanied by improved patient functioning in order for change to be considered clinically relevant in patients with schizNegative symptom improvement must be accompanied by improved patient functioning in order for change to be considered clinically relevant in patients with schiz
more…