REAGILA’s Effects on Primary and Secondary Negative Symptoms: Pseudospecificity
- Cariprazine is effective in both positive and negative symptoms of schizophrenia.1,5-7
- As shown in the cariprazine negative symptom study, negative symptom control with cariprazine is not attributed to an improvement in positive symptom control1
- Negative symptom control is also not attributed to an improvement in depressive symptom control1
- Negative symptom control is also not attributed to an improvement in EPS1
In this section
What is the Pseudospecificity Problem?
Although cariprazine is the first and only antipsychotic to demonstrate efficacy against another antipsychotic in a large-scale trial specially designed to evaluate patients with schizophrenia with predominant, persistent negative symptoms1, several antipsychotics have shown efficacy against negative symptoms versus placebo or other antipsychotics in trials of patients with general symptoms of schizophrenia2,8.
So, What’s the Difference?
Unfortunately, improvement in negative symptoms that occurs during an exacerbation of schizophrenia is ambiguous and does not demonstrate a genuine negative symptom treatment effect. The reason loops back around to the distinction between primary (ie, intrinsic to schizophrenia) and secondary (ie, occurring because of or caused by other factors; can respond to treatment of the underlying cause) negative symptoms. Including patients with secondary negative symptoms in clinical trials leads to complications and misinterpretation of what is or is not a direct therapeutic effect on negative symptoms. If patients aren’t stable and secondary negative symptoms are present, it is possible that apparent improvement in negative symptoms is in reality due to concurrent improvement in symptoms from other domains (ie, depression, psychosis, extrapyramidal symptoms) and not from improvement in negative symptoms themselves3.
This conundrum is referred to as pseudospecificity.


How Can Genuine Negative Symptom Treatment Effects Be Demonstrated?
A well-designed trial to evaluate negative symptoms in schizophrenia should demonstrate that an improvement in negative symptoms is not related to improvement in other domains of psychopathology that can affect ratings of negative symptoms4. This can be accomplished through the use of specific inclusion and exclusion criteria and outcome measures that evaluate changes in symptoms that could account for pseudospecific change.
In the negative symptom trial of cariprazine versus risperidone1, adult patients (18-65 years) with a diagnosis of schizophrenia and confirmed predominant negative symptoms and low levels of positive symptoms could participate8. Stringent inclusion and exclusion criteria had to be satisfied to ensure that change in negative symptoms was genuine and not the result of a pseudospecific effect1.
| General Inclusion Criteria1 |
| Patients had to be in a stable condition for at least 6 months before screening (ie, no psychiatric hospital admissions, acute exacerbations, or imprisonments) |
| Predominant negative symptoms for at 6 months (based on medical records or investigator judgment) |
| PANSS factor score for negative symptoms (PANSS-FSNS) score ≥24 at screening and during lead-in |
| Score ≥4 at on at least 2 of 3 core negative PANSS items (blunted affect, passive or apathetic social withdrawal, lack of spontaneity, and flow of conversation) |
| PANSS-FSNS score that diverged less than 25% from the screening score during a lead-in period |
| General Exclusion Criteria |
| Unstable condition |
| PANSS factor score for positive symptoms (PANSS-FSPS) ≥19 |
| PANSS-FSPS score increase of 25% or more during a lead-in period |
| Specific Exclusion Criteria for Pseudospecificity |
| Positive symptoms: score ≥4 on 2 or more positive PANSS items: delusions, hallucinatory behavior, grandiosity, suspiciousness, or unusual thought content |
| Moderate or severe depressive symptoms: Calgary Depression Scale for Schizophrenia (CDSS) total score >6 |
| Clinically relevant parkinsonism: investigator judged or score >3 on the sum of the first eight items of the Simpson-Angus Scale [SAS] |
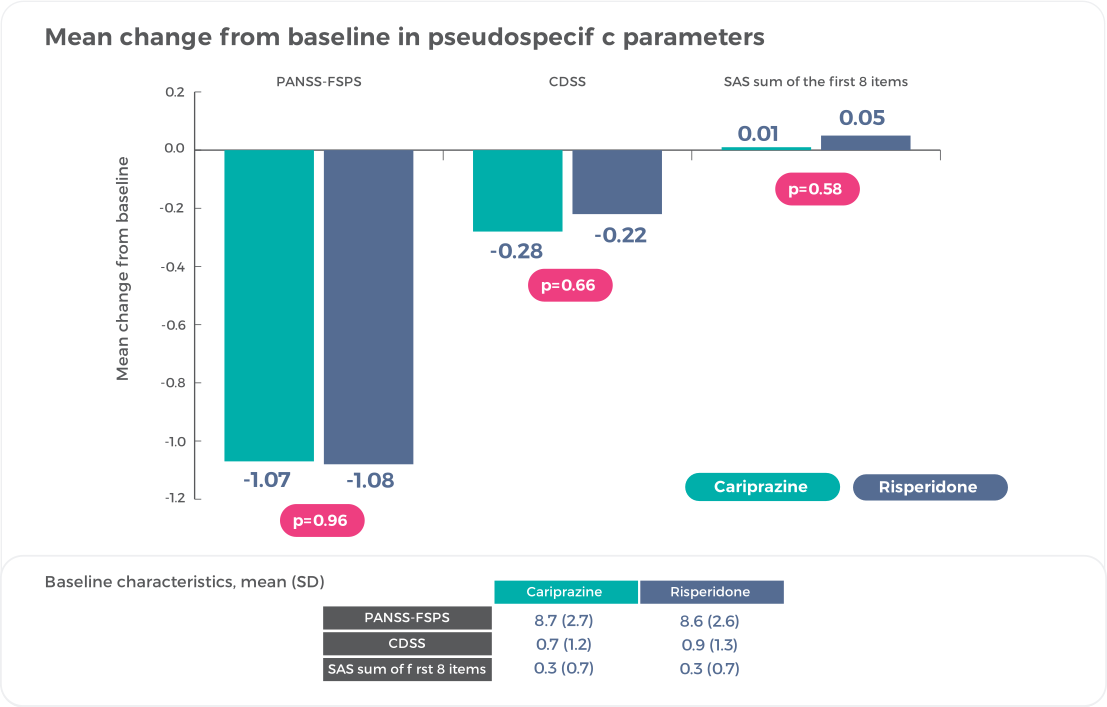
Real Negative Symptom Improvement = Small Change on Pseudospecificity Measures1
No differences were observed between cariprazine and risperidone on change from baseline in PANSS-FSPS, demonstrating that results on negative symptoms are not due to positive symptom improvement8.
Reference: Németh, G. et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet 389, 1103–13 (2017)1 (Graphic elaboration from text data)
Collectively, these results exclude indirect effects related to positive, depressive, or extrapyramidal symptom improvement as a factor in negative symptom improvement, indicating that negative symptom improvement with cariprazine was not a pseudospecific effect8.
References
- Németh, G. et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet 389, 1103–1113 (2017).
- Krause, M. et al. Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: a systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 268, 625–639 (2018).
- Buchanan, R. W., Breier, A., Kirkpatrick, B., Ball, P. & Carpenter, W. T. Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. Am. J. Psychiatry 155, 751–760 (1998).
- Marder, S. R., Daniel, D. G., Alphs, L., Awad, A. G. & Keefe, R. S. E. Methodological issues in negative symptom trials. Schizophr. Bull. 37, 250–254 (2011).
- Durgam, S. et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: A phase II, randomized clinical trial. Schizophr. Res. 152, 450–457 (2014).
- Kane, J. M. et al. Efficacy and Safety of Cariprazine in Acute Exacerbation of Schizophrenia: Results from an International, Phase III Clinical Trial. J. Clin. Psychopharmacol. 35, 367–373 (2015).
- Durgam, S. et al. Cariprazine in acute exacerbation of schizophrenia: A fixed-dose, phase 3, randomized, double-blind, placebo- and active-controlled trial. J. Clin. Psychiatry 76, e1574-82 (2015).
- Reagila SmPC.
Lancet
Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial.
REAGILA CONTROLS NEGATIVE SYMPTOMSOUR PRODUCT ON NEGATIVE SYMPTOMS
Negative symptoms of schizophrenia can occur as primary symptoms that are part of the underlying pathophysiology of schizophrenia or as secondary symptoms that Negative symptoms of schizophrenia can occur as primary symptoms that are part of the underlying pathophysiology of schizophrenia or as secondary symptoms that
more…REAGILA AND DAILY FUNCTIONINGOUR PRODUCT AND DAILY FUNCTIONING
Negative symptom improvement must be accompanied by improved patient functioning in order for change to be considered clinically relevant in patients with schizNegative symptom improvement must be accompanied by improved patient functioning in order for change to be considered clinically relevant in patients with schiz
more…