REAGILA in Patients with Acute Exacerbation of Schizophrenia
- Cariprazine (REAGILA® [EU], Vraylar® [US]) is an EMA- and FDA-approved medication that is effective for the treatment of acute symptoms of schizophrenia1,2.
- Cariprazine is effective in addressing aggression and hostility in patients with acute symptoms of schizophrenia3.
In this section
Why Start with Cariprazine?
Once the diagnosis of schizophrenia is made, clinicians, patients, and families have important treatment decisions to make. Although it seems intuitive to say that symptoms will improve only if patients take their medications as they were prescribed, the percentage of patients who don’t adhere to their antipsychotic regimen in schizophrenia is high (20% to 56%)4,5. To improve the chance of success, a patient should be given a treatment that has proven efficacy, good safety and tolerability, and a convenient administration schedule. Response, tolerability, and convenience can improve a drug’s acceptability, which may in turn help a patient stay on their treatment program6 and improve the opportunity for recovery.
The advantages of initiating treatment with Cariprazine include its broad-spectrum efficacy in short and long term7, in negative symptoms8, its good cardiometabolic profile9, low incidences of weight gain9 and sedation10, and convenient once-a-day dosing, with or without food1. This makes cariprazine a good treatment option for the treatment of schizophrenia symptoms.
Short-Term Efficacy
Evidence for the efficacy of Cariprazine in acute exacerbation of schizophrenia comes from 3 positive clinical studies in adult patients11-13. Each study consisted of a washout period (up to 1 week), 6 weeks of double-blind treatment, and a 2-week safety follow-up; fixed and fixed/flexible Cariprazine doses of 1.5 mg/d to 9 mg/d were evaluated across the studies; although the maximum approved dose of cariprazine is 6mg/day1. The primary endpoint in each study was change from baseline to week 6 in Positive and Negative Syndrome Scale (PANSS) total score; the secondary endpoint in each study was change from baseline to week 6 in the Clinical Global Impressions-Severity of Illness Scale (CGI-S)11-13.
Across 3 short-term pivotal studies, Cariprazine demonstrated efficacy versus placebo in adult patients with acute exacerbation of schizophrenia11-13. The efficacy of Cariprazine is comparable to other antipsychotics considering the trend toward increasing placebo effect over the past years9.
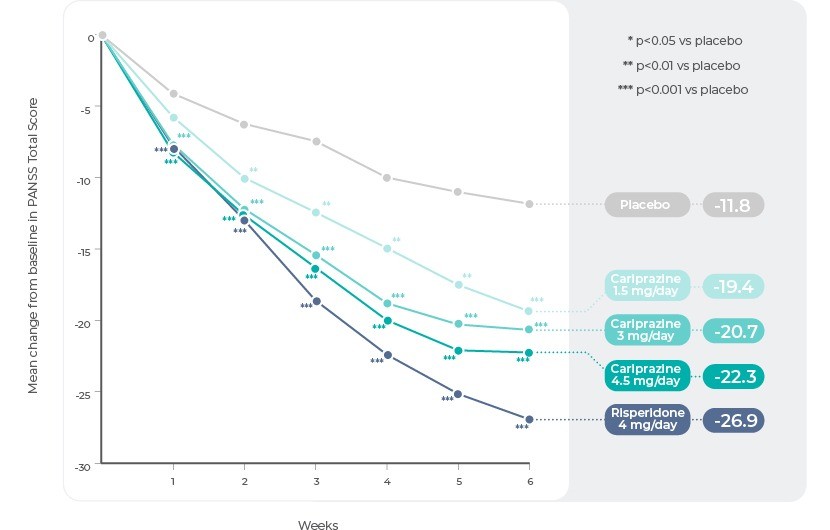
Study 111 (ClinicalTrials.gov Identifier: NCT00694707)
Study 1 was a phase 2b, multinational, multicentre, randomized, double-blind, placebo and active-controlled, parallel-group, fixed-dose study conducted from June 2008 to August 2009. Patients were randomized (1:1:1:1:1) to receive once-daily placebo (n=151), Cariprazine 1.5 mg (n=145), Cariprazine 3.0 mg/day (n=147), Cariprazine 4.5 mg (n=148), or risperidone 4.0 mg (included to confirm assay sensitivity, n=141).
The difference in change from baseline to week 6 in PANSS total score (primary efficacy) and CGI-S score (secondary efficacy) was statistically significant in favour of all Cariprazine doses (1.5, 3, and 4.5 mg/d) versus placebo. Risperidone was also statistically different than placebo on both efficacy parameters. Separation from placebo, which occurred early in the course of treatment for each Cariprazine dose (week 1 for Cariprazine 3 and 4.5 mg, week 2 for Cariprazine 1.5 mg), persisted through the end of the study. Although significant improvement versus placebo was observed for the lowest Cariprazine dose (1.5 mg/d) tested, greater treatment effects were observed with higher doses (3.0 mg/d and 4.5 mg/d).
Study 1: Change in PANSS Total Score by Week.
Reference: Adapted from Durgam, S. et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: A phase II, randomized clinical trial. Schizophr. Res. 152, 450–457 (2014).11
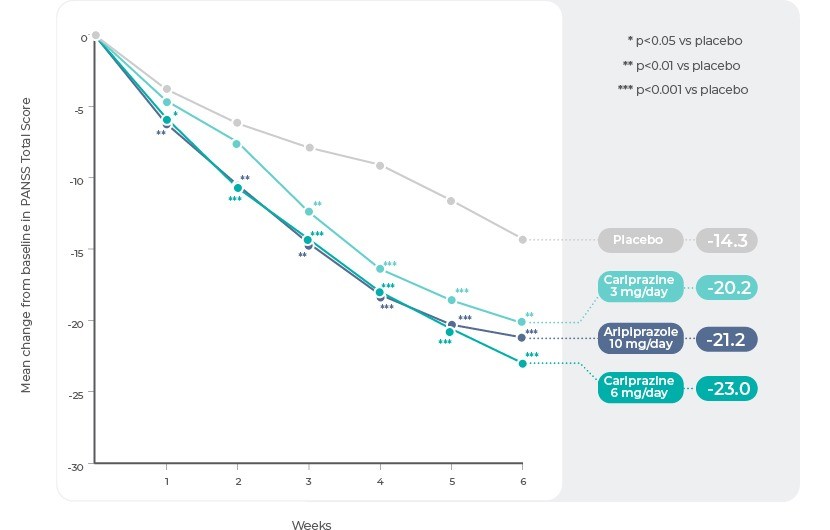
Study 213 (ClinicalTrials.gov Identifier: NCT01104766)
Study 2 was a phase 3 multicentre, multinational, randomized, double-blind, placebo and active-controlled, parallel-group, fixed-dose study conducted from April 2010 to December 2011. Patients were randomized (1:1:1:1) to receive once-daily treatment with placebo (n=153), Cariprazine 3 mg (n=155), Cariprazine 6 mg (n=157), or aripiprazole 10 mg (included to confirm assay sensitivity, n=152).
The difference in change from baseline to week 6 in PANSS total score (primary efficacy) and CGI-S score (secondary efficacy) was statistically significant in favour of each Cariprazine dose versus placebo. Aripiprazole was also statistically different than placebo on both efficacy parameters. Statistically significant separation from placebo was achieved at week 1 and week 3 in the Cariprazine 6 and 3 mg/d groups, respectively, and it was maintained through the end of the study in both dose groups.
Study 2: Change in PANSS Total Score by Week.
Reference: Adapted from Durgam, S. et al. Cariprazine in acute exacerbation of schizophrenia: A fixed-dose, phase 3, randomized, double-blind, placebo- and active-controlled trial. J. Clin. Psychiatry 76, e1574-82 (2015).13
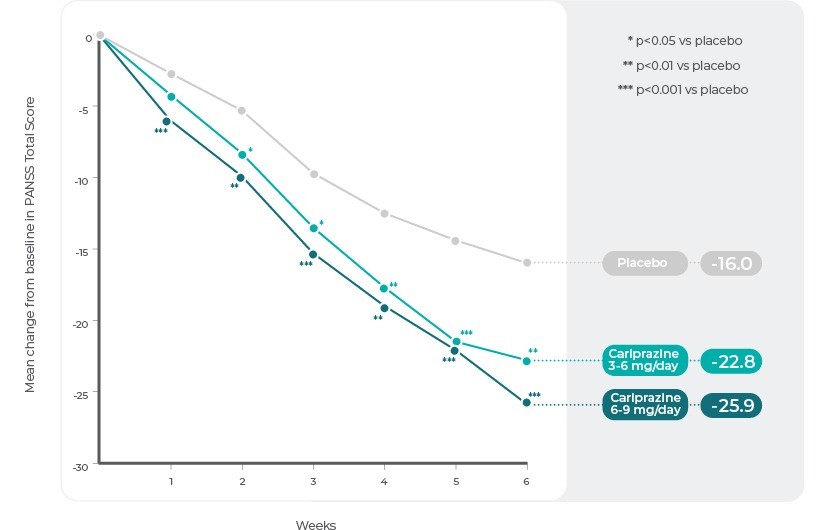
Study 312 (ClinicalTrials.gov Identifier: NCT01104779)
Study 3 was a phase 3 multicentre, multinational, randomized, double-blind, placebo-controlled, parallel-group, fixed/flexible-dose study conducted from April 2010 to December 2011. Patients were randomized (1:1:1) to receive once-daily treatment with Cariprazine 3-6 mg (n=151), Cariprazine 6-9 mg (n=148), or placebo (n=147). Cariprazine doses were gradually up-titrated over the first 2 weeks of the study to 3 mg (3-6 mg/d group) or 6 mg/d (6-9 mg/d group); in cases of inadequate response, the Cariprazine dose could be gradually increased to a maximum of 6 mg (3-6 mg/d group) or 9 mg (6-9 mg/d group) beginning at the end of week 2. The dosage was fixed from the end of week 3 to week 6.
The difference in change from baseline to week 6 in PANSS total score (primary efficacy) and CGI-S score (secondary efficacy) was statistically significant in favour of each Cariprazine group versus placebo. Statistical separation from placebo was detected beginning at week 1 in the Cariprazine 6-9 mg/d group and at week 2 in the 3-6 mg/d group; statistical differences were maintained until the end of the study in both cariprazine dose groups.
Study 3: Change in PANSS Total Score by Week.
Reference: Adapted from Kane, J. M. et al. Efficacy and Safety of Cariprazine in Acute Exacerbation of Schizophrenia: Results from an International, Phase III Clinical Trial. J. Clin. Psychopharmacol. 35, 367–373 (2015).12
Managing Hostility
Aggressive behaviours in schizophrenia can be caused by diverse factors including substance abuse, medication noncompliance, mistreatment in childhood, conduct disorder, and economic difficulties14. Most patients with schizophrenia are not aggressive, but they are at an increased risk of developing hostile or aggressive behaviours15,16. Hostility, which is characterized by irritability, anger, resentment, or aggression, is not the same as violent behaviour itself, but it has the potential to escalate to violence17. Hostile and aggressive behaviour are related to the high burden of illness in schizophrenia through increased hospitalizations, stigma, involvement with the criminal justice system, and physical vulnerability as a result of fights or altercations18. Atypical antipsychotics are a valuable treatment option for patients with schizophrenia accompanied by hostility19.
Effects of Cariprazine on Hostility
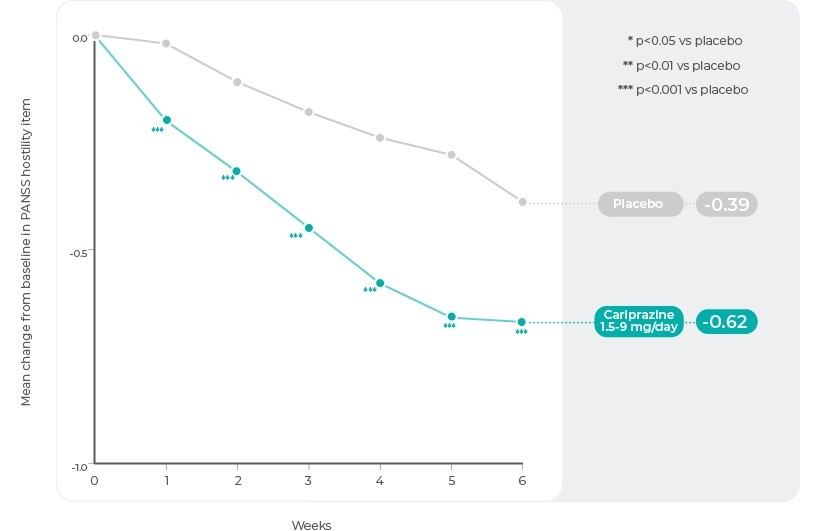
A pooled post hoc analysis of data from the 3 positive acute studies of Cariprazine in patients with acute exacerbation of schizophrenia was conducted to evaluate the effect of Cariprazine (n=1024) versus placebo (n=442) on a measure of hostility; dose groups were combined (1.5-9 mg/d) for post hoc analysis3. Hostility was measured as mean change from baseline to week 6 on the PANSS hostility item (P7), which assesses verbal and nonverbal expressions of anger and resentment, including sarcasm, passive-aggressive behaviour, verbal abuse, and assaultiveness20. Scores range from 1 (no hostility) to 7 (extreme hostility including marked anger, extreme uncooperativeness, or physical assault)20.
Mean change from baseline on the PANSS hostility item was assessed in the overall pooled patient population and in subgroups of patients categorized by increasing levels of baseline hostility: minimally severe (item score ≥2: questionable pathology/upper extreme of normal); mildly severe (item score ≥3: indirect or restrained communication of anger including sarcasm, disrespect, hostile expressions, occasional irritability); or moderately severe (item score ≥4: overtly hostile attitude, frequent irritability, direct expression of anger or resentment). To test the specific anti-hostility effect of Cariprazine, separate analyses were conducted to correct for possible confounding effects of change in positive symptoms or sedation3.
In the overall pooled population, the difference in change from baseline to week 6 on the PANSS hostility item analysis was statistically significant in favour of Cariprazine at every analysis point from week 1 through week 6. The additional analyses that adjusted for positive symptoms and sedation further demonstrated that the effect of Cariprazine on hostility was partially independent of improvement in PANSS positive symptoms and independent of the presence or absence of sedation3.
PANSS Hostility Item: Change by Week in the Overall Pooled Population3
Reference: Adapted from Citrome, L., Durgam, S., Lu, K., Ferguson, P. & Laszlovszky, I. The effect of Cariprazine on hostility associated with schizophrenia: Post hoc analyses from 3 randomized controlled trials. J. Clin. Psychiatry 77, 109–115 (2016).3
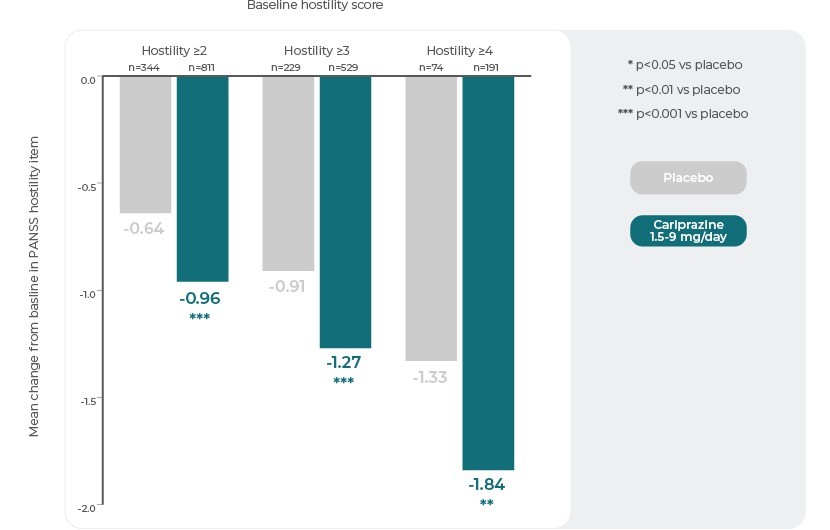
In the baseline hostility subgroup analyses, statistically significant differences in mean change from baseline to week 6 were seen in favour of Cariprazine versus placebo in each of the baseline hostility cohorts analysed. At week 6, higher levels of baseline hostility were associated with greater magnitude of change in the hostility item, with the greatest effect seen in Cariprazine patients with baseline hostility ≥43.
PANSS Hostility Item: Change From Baseline in Severity Subgroups3
Reference: Adapted from Citrome, L., Durgam, S., Lu, K., Ferguson, P. & Laszlovszky, I. The effect of Cariprazine on hostility associated with schizophrenia: Post hoc analyses from 3 randomized controlled trials. J. Clin. Psychiatry 77, 109–115 (2016).3
This post hoc analysis suggests that Cariprazine may have specific anti-hostility effects that are independent of a general antipsychotic effect and sedation, and greater in patients with higher levels of baseline hostility3. Cariprazine may be a good treatment option for patients with acute schizophrenia and hostile behaviour.
References
- Reagila SmPC.
- FDA. Vraylar Label. (2017).
- Citrome, L., Durgam, S., Lu, K., Ferguson, P. & Laszlovszky, I. The effect of Cariprazine on hostility associated with schizophrenia: Post hoc analyses from 3 randomized controlled trials. J. Clin.
- Lacro, J. P., Dunn, L. B., Dolder, C. R., Leckband, S. G. & Jeste, D. V. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: A comprehensive review of recent literature. J. Clin. Psychiatry 63, 892–909 (2002).
- Novick, D. et al. Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res. 176, 109–113 (2010).
- Martin, L. R., Williams, S. L., Haskard, K. B. & DiMatteo, M. R. The challenge of patient adherence. Ther Clin Risk Manag. 1, 189–199 (2005).
- Durgam, S. et al. Long-term cariprazine treatment for the prevention of relapse in patients with schizophrenia: A randomized, double-blind, placebo-controlled trial. Schizophr. Res. 176, 264–271 (2016).
- Németh, G. et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet 389, 1103–1113 (2017).
- Leucht, S. et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: Systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am. J. Psychiatry 174, 927–942 (2017).
- Citrome, L. Activating and sedating adverse effects of second-generation antipsychotics in the treatment of schizophrenia and major depressive disorder: Absolute risk increase and number needed to harm. J. Clin. Psychopharmacol. 37, 138–147 (2017).
- Durgam, S. et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: A phase II, randomized clinical trial. Schizophr. Res. 152, 450–457 (2014).
- Kane, J. M. et al. Efficacy and Safety of Cariprazine in Acute Exacerbation of Schizophrenia: Results from an International, Phase III Clinical Trial. J. Clin. Psychopharmacol. 35, 367–373 (2015).
- Durgam, S. et al. Cariprazine in acute exacerbation of schizophrenia: A fixed-dose, phase 3, randomized, double-blind, placebo- and active-controlled trial. J. Clin. Psychiatry 76, e1574-82 (2015).
- Volavka, J. & Citrome, L. Pathways to aggression in schizophrenia affect results of treatment. Schizophr. Bull. 37, 921–929 (2011).
- Volavka, J. et al. Efficacy of antipsychotic drugs against hostility in the European First-Episode Schizophrenia Trial (EUFEST). J. Clin. Psychiatry 72, 955–961 (2011).
- Walsh, E., Buchanan, A. & Fahy, T. Violence and schizophrenia: Examining the evidence. Br. J. Psychiatry 180, 490–495 (2002).
- Witt, K., van Dorn, R. & Fazel, S. Risk Factors for Violence in Psychosis: Systematic Review and Meta-Regression Analysis of 110 Studies. PLoS One 8, e55942 (2013).
- Ahmed, A. O., Hunter, K. M., van Houten, E. G., Monroe, J. M. & Bhat, I. A. Cognition and other targets for the treatment of aggression in people with schizophrenia. Ann Psychiatry Ment Heal. 2, 1004 (2014).
- Krakowski, M. I., Czobor, P., Citrome, L., Bark, N. & Cooper, T. B. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch. Gen. Psychiatry 63, 622–629 (2006).
- Kay, S. R. Positive and negative syndromes in schizophrenia: Assessment and research. (Psychology Press, 1991).
CNS Drugs
Cariprazine: A review in schizophrenia.
REAGILA’S BENEFITS IN THE LONG TERMOUR PRODUCT’S BENEFITS IN THE LONG …
Atypical antipsychotics need to be effective across the span of illness in patients with schizophrenia. When patients experience acute exacerbation of psychoticAtypical antipsychotics need to be effective across the span of illness in patients with schizophrenia. When patients experience acute exacerbation of psychotic
more…REAGILA CONTROLS NEGATIVE SYMPTOMSOUR PRODUCT ON NEGATIVE SYMPTOMS
Negative symptoms of schizophrenia can occur as primary symptoms that are part of the underlying pathophysiology of schizophrenia or as secondary symptoms that Negative symptoms of schizophrenia can occur as primary symptoms that are part of the underlying pathophysiology of schizophrenia or as secondary symptoms that
more…