Neurochemical Basis of Schizophrenia
- The dopamine hypothesis continues to be a valid neurobiological theory in schizophrenia1,2.
- Dopamine receptors relevant to schizophrenia psychopathology have differential distribution patterns, affinities and characteristics in the human brain.
- In schizophrenia, positive symptoms are thought to be associated with a hyperdopaminergic state in the mesolimbic system whereas a hypodopaminergic state in the mesocortical system has been associated with negative and cognitive symptoms.
In this section
The Dopamine Hypothesis
The dopamine hypothesis of schizophrenia began as a theory based on circumstantial evidence obtained from clinical observations following antipsychotic treatment, and has advanced to the present state, where imaging studies can provide direct testing and validation, allowing for a better understanding of the underlying pathophysiological mechanisms in schizophrenia3.
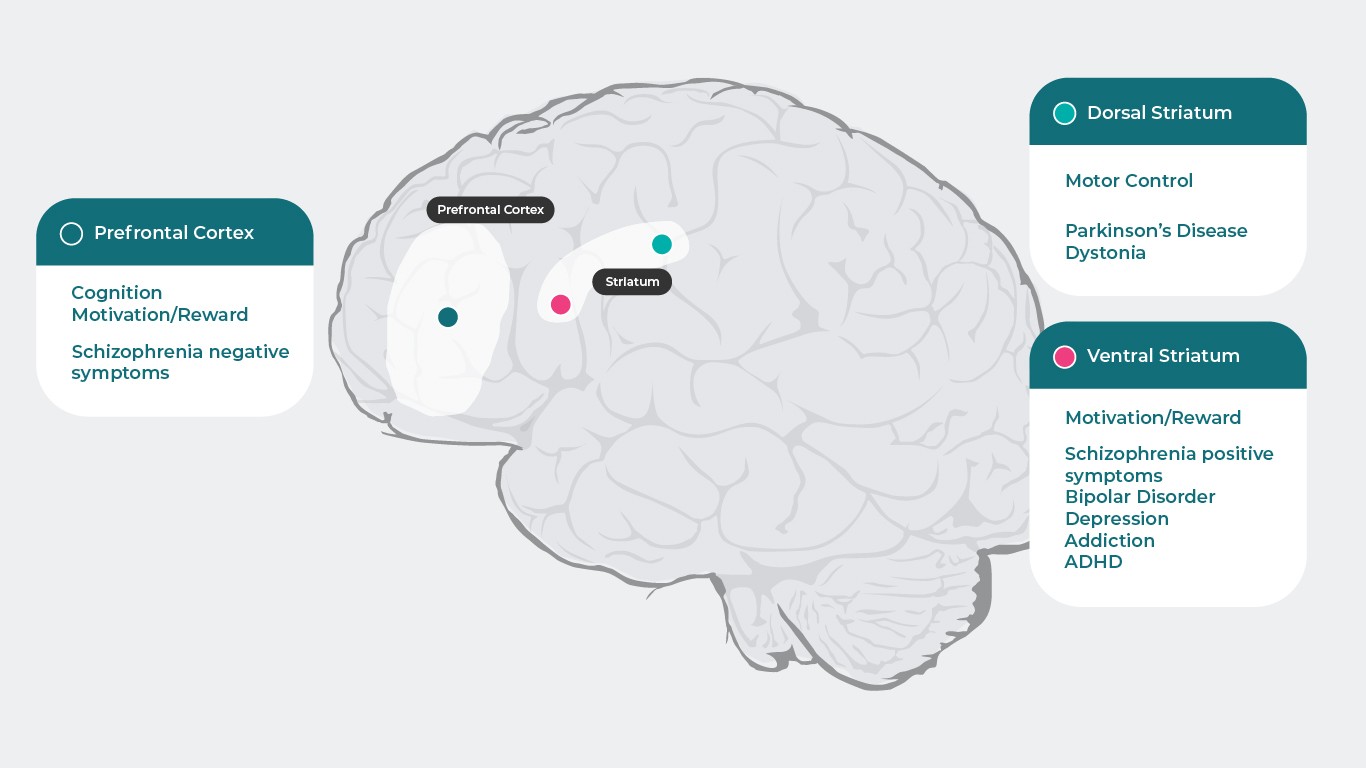
Dopamine and The Living Brain
Because dopamine has specific actions at each receptor subtype, which are responsible for wide range of psychiatric and neurologic functions (Prefrontal Cortex: Cognition5, Motivation/Reward6–10, Schizophrenia negative symptoms11, Dorsal Striatum: Motor Control12, Parkinson’s Disease1, Dystonia1
Ventral Striatum: Motivation/Reward6–10, Schizophrenia positive symptoms11,13, Bipolar Disorder14, Depression8, Addiction15, ADHD16), disruption of these processes can lead to numerous symptoms associated with psychiatric disease. Psychotropic medications that have activity at dopamine receptors can improve many of these symptoms, though a better understanding of dopamine function across different brain regions and the mechanisms underlying schizophrenia is needed to fully comprehend how antipsychotics target dopamine neurotransmission and treat schizophrenia4.
References: Adapted from Stahl SM. Essential Pharmacology 4th edition 20131 ; Cools R, D’Esposito M. Biol Psychiatry. 2011;69(12):e113-125.5; Hamid AA, et al. Nat Neurosci. 2016;19(1):117-126.6; Koob GF, et al. NIDA Res Monogr. 1994;145:1–187; Biesdorf C, et al. Neurobiol Learn Mem. 2015;123:125-1398; Der-Avakian A, et al. Trends Neurosci. 2012 Jan;35(1):68-77.9; Leggio GM, et al. Eur Neuropsychopharmacol. 2008 Apr;18(4):271-7.10; Stahl SM. Prim Care Companion J Clin Psychiatry.2003;5(3)9-1311; Plowman EK, et al. J Parkinsons Dis. 2011;1(1):93-10012; Dichter GS, et al. J Neurodev Disord. 2012;4:19513; Ashok AH, et al. Mol Psychiatry.2017 May;22(5):666–67914; Orio L, et al. Addict Biol. 2010;15(3):312-32315; Volkow ND, et al. J Neurosci. 2012;32(3):841-84916.
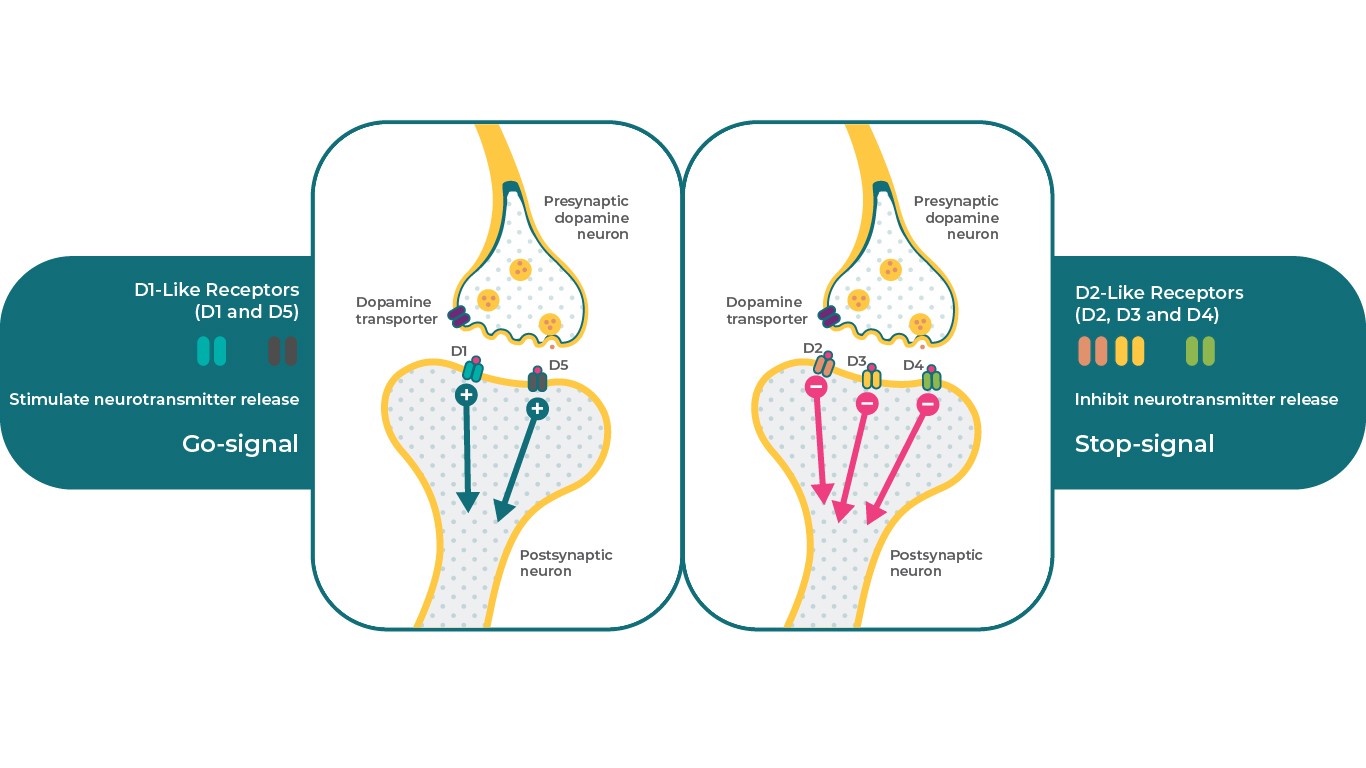
Dopamine Receptor Subtypes
There are 5 types of dopamine receptors which can be divided into 2 major classes. The D1-like family of receptors include the dopamine D1 and D5 receptors, whereas the D2-like family consists of the D2, D3, and D4 receptors. From a clinical perspective, dopamine D2, D3, and D1 are generally considered the most relevant receptors to schizophrenia pathophysiology. Depending on receptor subtype, dopamine receptors can exert differential effects on the same secondary messenger system with one receptor serving as a “stop” signal, while different subtypes can serve as a “go” signal4. For example, D1 receptors can stimulate cAMP, while D2 and D3 receptors can inhibit this secondary messenger.
In this way, dopamine D1, D2, and D3 receptors can serve very specific but differential neurobiological functions in the presence of dopamine, which allows one neurotransmitter to control diverse physiological, behavioural, and pathological conditions4,17.
References: Adapted from Stahl, S. M. Dazzled by the dominions of dopamine: Clinical roles of D3, D2, and D1 receptors. CNS Spectr. 22, 305–311 (2017)4
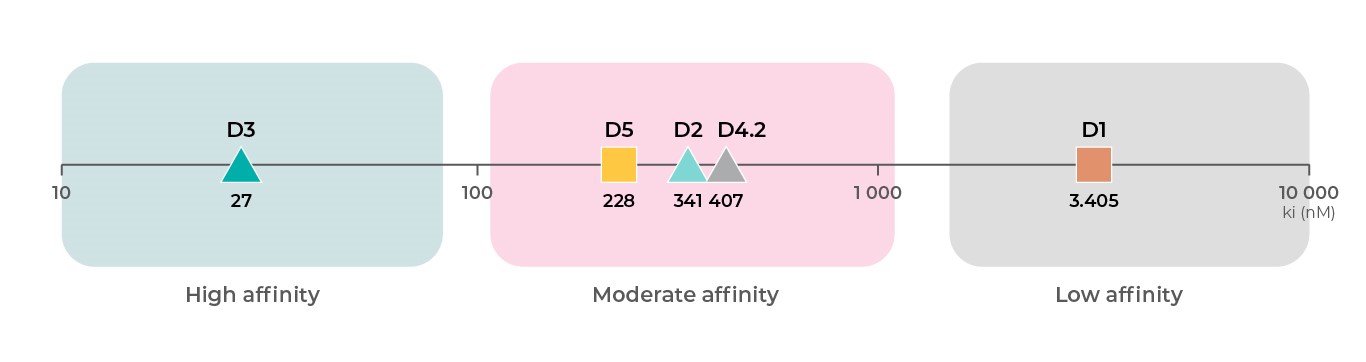
Dopamine Receptor Affinities
In addition to differential functions across dopamine receptor subtypes, each dopamine receptor subtype has a different affinity for dopamine. Based on binding data from the PDSP Ki database, D3 receptors19 have the highest affinity for dopamine, followed by D219,20, D421, and D518 receptors at intermediate affinities, and then D118,19 receptors with the lowest affinity for dopamine4 .
References: All binding data are from PDSP Ki database.
Affinity to D1 subtype: Toll L, NIDA Res Monogr 1998, 178:44046619 ; Sunahara RK, Nature 1991, 350:614-61918
Affinity to D2 subtype: Michaelides MR 1995, J Med Chem 38:3445-344720; Toll L, NIDA Res Monogr 1998, 178:44046619
Affinity to D3 subtype: Toll L, NIDA Res Monogr 1998, 178:44046619; Cussac D, Naunyn Schmied Arch Pharmacol 2000, 361:569-572;
Affinity to D4 subtype: Tallman JF, J Pharm Exp Ther 1997, 282:1011-101921;
Affinity to D5 subtype: Sunahara RK, Nature 1991, 350:614-61918; Stahl SM, CNS Spect 2016, 21:123-12722.
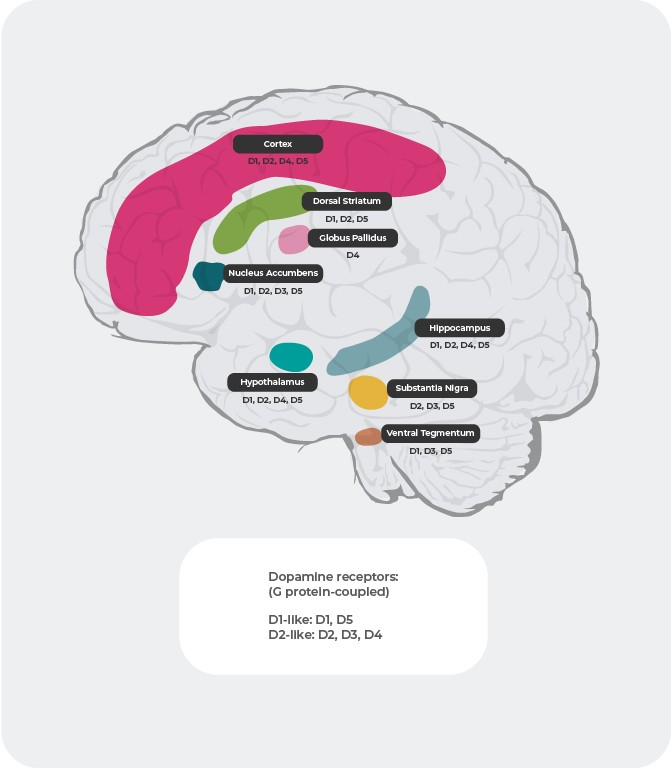
Dopamine Receptor Distributions4,22-27
Beyond different affinities, the distribution of dopamine receptors differs between subtypes in the brain. For example, overactive dopamine release at D2 postsynaptic receptors in the ventral striatum is purported to cause positive symptoms of psychosis. As a result, dopamine D2 receptors represent the primary target of most antipsychotic drugs used to treat schizophrenia. D3 receptors are highly expressed in the limbic areas, hypothalamus, and ventral tegmental area/substantia nigra, areas thought to be involved with the regulation of cognition, mood, and motivation. In the prefrontal cortex, where the expression of dopamine D2 and D3 receptors are sparse, dopamine neurotransmission is more heavily controlled postsynaptic D1 receptors.
References: Adapted from Stahl, CNS spec. 2017;22:305-311 18; Fatemi SH. The medical basis of psychiatry. 4th edition. Sringer 2016;p93-9427
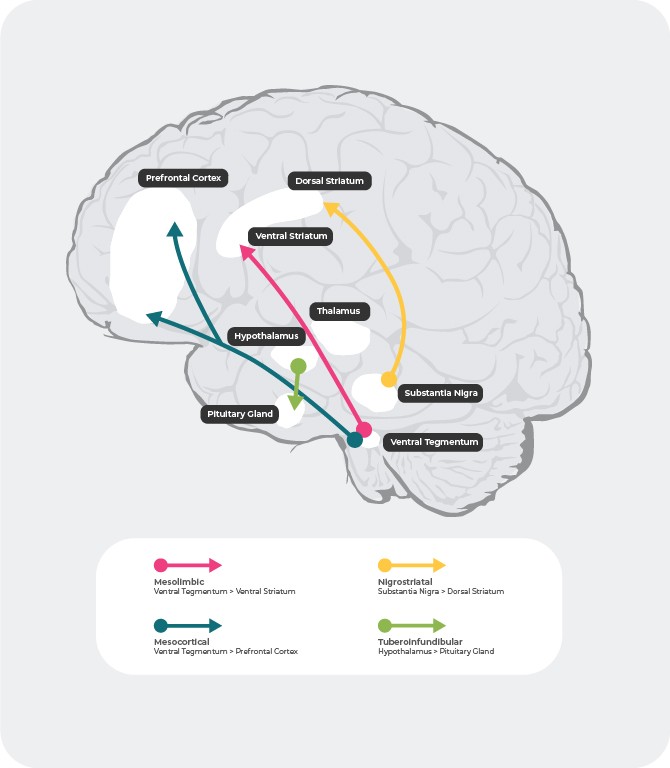
Dopaminergic Pathways
There are 4 major dopaminergic pathways in the human brain: the mesolimbic, mesocortical, nigrostriatal, and tuberoinfundibular pathways27:
mesolimbic pathway: the mesolimbic pathway, also known as the reward pathway, transmits dopamine from the ventral tegmental area (VTA) to the ventral striatum. The ventral tegmental area is located in the midbrain and the ventral striatum is located in the forebrain and includes both the nucleus accumbens and olfactory tubercle.
mesocortical pathway: the mesocortical pathway, which is believed to be involved in cognition and emotion, transmits dopamine from the ventral tegmental area to the prefrontal cortex.
nigrostriatal pathway: the nigrostriatal pathway, which is involved in the regulation of movement, transmits dopamine from the substantia nigra pars compacta (SNc), which is located in the midbrain, to the caudate nucleus and putamen, which are located in the dorsal striatum.
tuberoinfundibular pathway: the tuberoinfundibular pathway transmits dopamine from the infundibular nucleus of the hypothalamus to the pituitary gland. This pathway regulates secretion of pituitary gland hormones, including prolactin.
References: Adapted from Fatemi SH. The medical basis of psychiatry. 4th edition. Sringer 2016;p93-9427
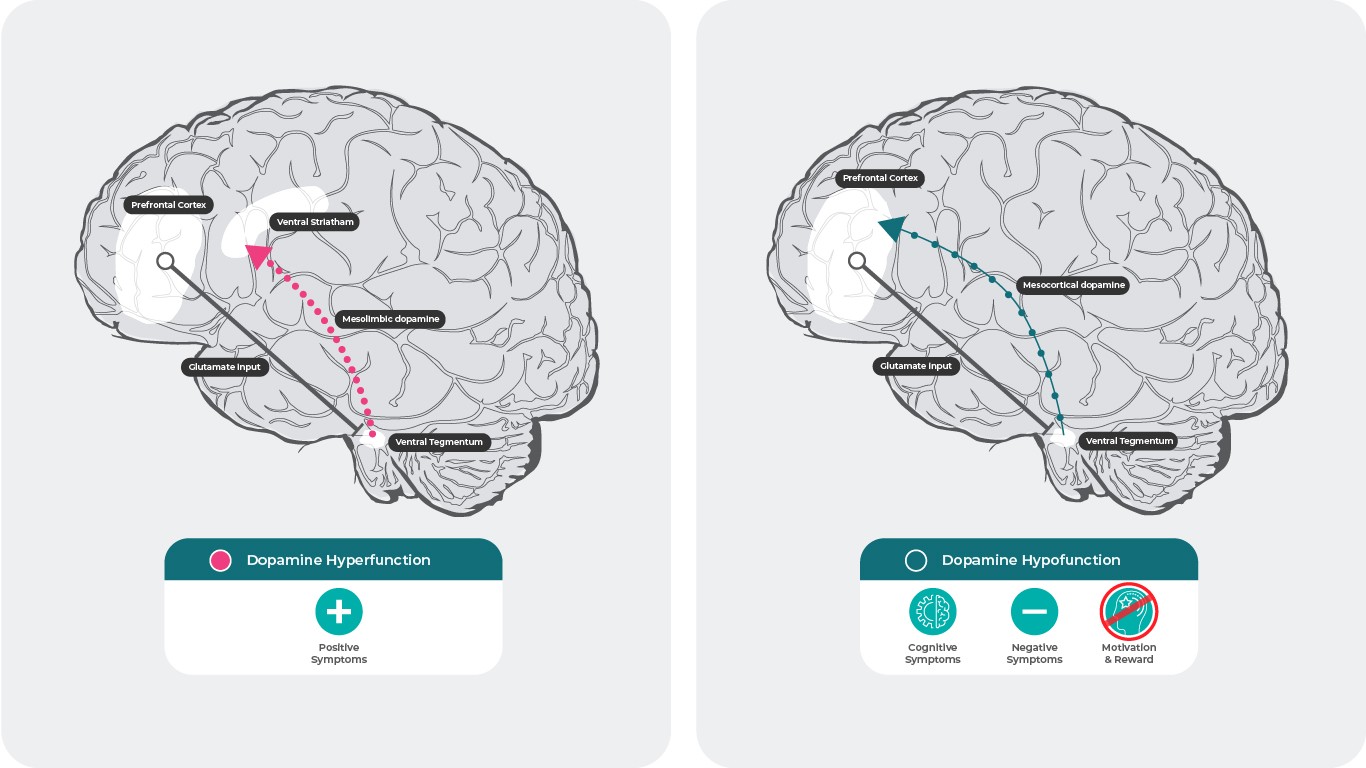
Dopamine and Schizophrenia28,29
The dopamine hypothesis of schizophrenia suggests that hyperactive dopamine neurotransmission in subcortical and limbic brain regions is linked to the positive symptoms of schizophrenia30, while hypofunctionality of mesocortical dopamine projections to the prefrontal cortex may be more responsible for negative and cognitive symptoms31,32.
References: Adapted from Stahl SM. 4th ed. New York, NY: Cambridge University Press; 20131; Howes J Psychopharmacol. 2015 February; 29(2): 97–115.; 35. Elert E, Nature 2014. 508. s2-s329
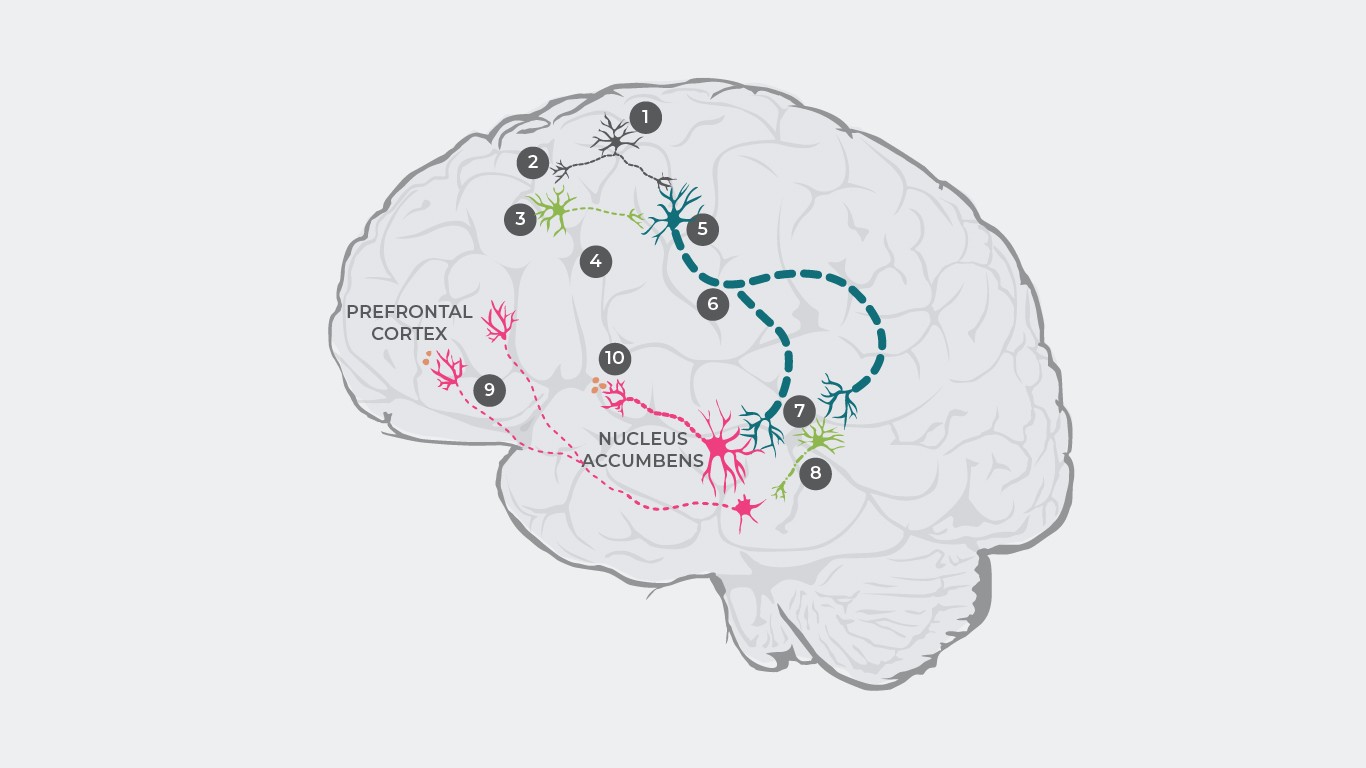
Several theories of dopamine dysregulation have been offered to explain the pathophysiology of schizophrenia33,34. For example, research has posited that schizophrenia pathophysiology may primarily be caused by a dysregulation of glutamate causing a dopaminergic disbalance35.
The cascade begins with glutamate neurons firing and sending out an electric pulse (1) which causes glutamate release at the synapse (2).
Under healthy conditions, glutamate then binds to receptors on inhibitory GABA neurons and excitatory glutamate neurons. In patients with schizophrenia, however, the binding of glutamate to GABA neurons is disturbed (3), resulting in inhibitory neurotransmitters not being released (4). However, glutamate will still bind to excitatory glutamate neurons, and in the absence of inhibitory GABA neurons (5), an increased signal will be sent to the brainstem (6).
As a consequence, too much glutamate is released at the synapse, overstimulating both dopamine neurons and other inhibitory GABA neurons (7).
Overstimulated GABA neurons overinhibit a different dopamine neuron, thereby suppressing its activity (8). This underactive dopamine neuron carries intermittent signals from the ventral tegmental area to the prefrontal cortex where too little dopamine is released (9), resulting in hypodopaminergic state in the mesocortical system which in turn results in the manifestation of negative symptoms1.
Subsequently, these overstimulated dopamine neurons carry increased signals from the ventral tegmental area to the ventral striatum, causing too much dopamine to be released there (10), resulting in hyperdopaminergia in the mesolimbic system and causing positive symptoms’.
References: Adapted from Stahl SM. 4th ed. New York, NY: Cambridge University Press; 20131; Howes J Psychopharmacol. 2015 February; 29(2): 97–11529; Elert Nature volume 508, pages S2–S3 (03 April 2014)35;
Research supports that increased dopamine D2 receptor neurotransmission in subcortical and limbic regions in the brain contributes to positive symptoms31,32. However, the mechanisms that give rise to negative and cognitive symptoms are not as clear.
Currently, it is assumed that decreased dopamine release in the prefrontal cortex is caused by activation of presynaptic dopamine D3 autoreceptors in the ventral tegmental area that project to the prefrontal cortex. Among dopamine receptor subtypes, D3 receptors have the highest affinity for dopamine. Unlike D1 or D2 receptors, D3 receptors can be stimulated even under conditions of low-level dopamine release, which can “tamper” fluctuation effects from bursts of phasic dopamine release. Blocking this attenuation disinhibits dopamine release and enhances neurotransmission. This increase in dopamine may reverse the hypodopaminergic state and result in improvements in negative symptoms and cognition via activation of D1 receptors in the prefrontal cortex, which are not active in low dopamine conditions32.
In animal models, including studies in primates, the most favourable cognitive effects are achieved when D1 receptor activity is optimized in the prefrontal cortex. Too much dopamine as well as too little dopamine activity at cortical D1 receptors are associated with cognitive dysfunction. Hypothetically, drugs that either block or overstimulate D1 receptors could dysregulate dopamine stimulation and contribute to cognitive, negative, and mood symptoms by causing a lack of harmony in neurotransmission at cortical synapses. It is also possible that D3 receptor antagonists could disinhibit dopamine release and harmonize cortical circuits, which in turn could result in improved regulation of cognition, mood, and negative symptoms4.
References
- Stahl, S. M. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. (Cambridge University Press, 2013).
- Seeman, P. Schizophrenia and dopamine receptors. Eur. Neuropsychopharmacol. 23, 999–1009 (2013).
- Kaar, S. J., Natesan, S., McCutcheon, R. & Howes, O. D. Antipsychotics: Mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology (2019). doi:10.1016/j.neuropharm.2019.107704
- Stahl, S. M. Dazzled by the dominions of dopamine: Clinical roles of D3, D2, and D1 receptors. CNS Spectr. 22, 305–311 (2017).
- Cools, R. & D’Esposito, M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 69, e113-125 (2011).
- Hamid, A. A. et al. Mesolimbic dopamine signals the value of work. Nat. Neurosci. 19, 117-126. (2016).
- Koob, G. F., Caine, B., Markou, A., Pulvirenti, L. & Weiss, F. Role for the mesocortical dopamine system in the motivating effects of cocaine. NIDA Res. Monogr. Ser. 145, 1-18. (1994).
- Biesdorf, C. et al. Dopamine in the nucleus accumbens core, but not shell, increases during signaled food reward and decreases during delayed extinction. Neurobiol. Learn. Mem. 123, 125–139 (2015).
- Der-Avakian, A. & Markou, A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 35, 68–77 (2012).
- Leggio, G. M., Micale, V. & Drago, F. Increased sensitivity to antidepressants of D3 dopamine receptor-deficient mice in the forced swim test (FST). Eur. Neuropsychopharmacol. 18, 271–277 (2008).
- Stahl, S. M. Describing an atypical antipsychotic: receptor binding and its role in pathophysiology. Prim. Care Companion J. Clin. Psychiatry 5, 9–13 (2003).
- Plowman, E. K., Thomas, N. J. & Kleim, J. A. Striatal dopamine depletion induces forelimb motor impairments and disrupts forelimb movement representations within the motor cortex. J. Parkinsons. Dis. 1, 93–100 (2011).
- Dichter, G. S., Damiano, C. A. & Allen, J. A. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: Animal models and clinical findings. J. Neurodev. Disord. 4, 19 (2012).
- Ashok, A. H. et al. The dopamine hypothesis of bipolar affective disorder: The state of the art and implications for treatment. Mol. Psychiatry 22, 666–679 (2017).
- Orio, L., Wee, S., Newman, A. H., Pulvirenti, L. & Koob, G. F. The dopamine D3 receptor partial agonist CJB090 and antagonist PG01037 decrease progressive ratio responding for methamphetamine in rats with extended-access. Addict. Biol. 15, 312–323 (2010).
- Volkow, N. D. et al. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J. Neurosci. 32, 841–849 (2012).
- Beaulieu, J. M., Espinoza, S. & Gainetdinov, R. R. Dopamine receptors – IUPHAR review 13. Br. J. Pharmacol. 172, 1–23 (2015).
- Sunahara, R. K. et al. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature 350, 614–619 (1991).
- Toll, L. et al. Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res. Monogr 178, 440–466 (1998).
- Michaelides, M. R. et al. (5aR, 11bS)-4,5,5a,6,7, 11b-hexahydro-2-propyl-3-thia-5-azacyclopent-1-ena[c]-phenanthrene-9,10-diol (A-86929): A potent and selective dopamine D1 agonist that maintains behavioral efficacy following repeated administration and characterization of its dia. J. Med. Chem. 38, 3445–3447 (1995).
- Tallman, J. F. et al. Ngd 94-1 – identification of a noval, high-affinity antagonist at the human dopamine D-4 receptor 1. J. Pharmacol Exp Ther 282, 1011–1019 (1997).
- Stahl, S. M. Mechanism of action of cariprazine. CNS Spectr. 21, 123–127 (2016).
- Gurevich, E. V. & Joyce, J. N. Distribution of dopamine D3 receptor expressing neurons in the human forebrain comparison with D2 receptor expressing neurons. Neuropsychopharmacology 20, 60–80 (1999).
- Mengod, G. et al. Visualization of dopamine D1, D2 and D3 receptor mRNA’s in human and rat brain. Neurochem. Int. 20, Suppl:33S-43S (1992).
- Meador-Woodruff, J. H. et al. Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology 15, 17–29 (1996).
- Fuxe, K. et al. Dopaminergic Systems in the Brain and Pituitary. in Basic and Clinical Aspects of Neuroscience (eds. Flückinger, E., Müller, E. E. & Thorner, M. O.) (Springer, 1985). doi:10.1007/978-3-642-69948-1_2
- Fatemi, S. H. & Clayton, P. J. The medical basis of psychiatry: Fourth edition. The Medical Basis of Psychiatry: Fourth Edition (2016). doi:10.1007/978-1-4939-2528-5
- Howes, O. D. & Kapur, S. The dopamine hypothesis of schizophrenia: Version III – The final common pathway. Schizophr. Bull. 35, 549–562 (2009).
- Howes, O., McCutcheon, R. & Stone, J. Glutamate and dopamine in schizophrenia: An update for the 21st century. J. Psychopharmacol. 29, 97–115 (2015).
- Desbonnet, L. Modeling the Psychopathological Dimensions of Schizophrenia. in Handbook of Behavioural Neuroscience 267–284 (2016).
- Toda, M. & Abi-Dargham, A. Dopamine hypothesis of schizophrenia: Making sense of it all. Curr. Psychiatry Rep. 9, 329–336 (2007).
- Stahl, S. M. Drugs for psychosis and mood: Unique actions at D3, D2, and D1 dopamine receptor subtypes. CNS Spectr. 22, 375–384 (2017).
- Grace, A. A. & Gomes, F. V. The circuitry of dopamine system regulation and its disruption in schizophrenia: Insights into treatment and prevention. Schizophr. Bull. 45, 148–157 (2019).
- Grace, A. A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 17, 524–532 (2016).
- Elert, E. Aetiology: Searching for schizophrenia’s roots. Nature 508, S2-3 (2014).
CNS Spectrums
Dazzled by the dominions of dopamine: clinical roles of D3, D2, and D1 receptors.
ANTIPSYCHOTICS, DIFFERENT MOAS?ANTIPSYCHOTICS, DIFFERENT MOAS?
The clinical efficacy profile of typical antipsychotic agents appears to depend on high affinity for and full antagonist activity at dopamine D2 receptors. Due The clinical efficacy profile of typical antipsychotic agents appears to depend on high affinity for and full antagonist activity at dopamine D2 receptors. Due
more…HOW DOES REAGILA WORK?HOW DOES OUR PRODUCT WORK?
Cariprazine has high affinity for dopamine D3 and D2 receptors as well as serotonin 5 HT2B and 5 HT1A receptors, moderate affinity to 5 HT2A, histamine H1, and Find out more about its mechanism of action
more…