The Safety and Tolerability Profile of REAGILA
- Overall, cariprazine has a good safety profile and is generally well tolerated1-3
- Good tolerability is an integral component of a drug’s overall effectiveness because patients who don’t take their medication because of side effects or other issues that affect adherence cannot benefit from it4
In this section
Clinical Studies in Schizophrenia – Basis for The Safety and Tolerability Profile of Cariprazine
The safety and tolerability of cariprazine was evaluated in all of the clinical studies in the cariprazine development program: a short-term, phase 2 exploratory study, three short-term, phase 3 studies, one long-term, relapse prevention study, two open-label safety studies and one negative symptom study5. The total number of cariprazine-treated patients who were included in the safety dataset was 2728; of these patients, 2048 patients were in the most relevant therapeutic dose range group of 1.5-6 mg/d5.
Excursion: Adverse Events vs. Side Effects
| In clinical drug trials, adverse events are all unpleasant (adverse) events that occur during the course of the study, independently of whether these are due to drug therapy or not. Adverse reactions (or side effects) are those unpleasant events that can be attributed to the drug itself. Usually for clinical trials, both types of events are examined and noted, as they both have value: While adverse reactions reflect the opinions of the treating physicians and therefore have clinical value, adverse events are a more general collection of events independently of personal judgement. The relevance of this difference can be demonstrated in an example: if in a clinical study 4 patients experience insomnia, the adverse event table would show 4/4. However the treating physician knows that 1 of these patients always works night shifts and has difficulties sleeping when on the dayshift – so for this patient he believes the insomnia is not related to the drug but to his work. So the adverse reaction table would show 3/4 patients. On a large scale this difference changes the incidence rates of certain events, and therefore it is worthwhile knowing which data is presented. |
Side effects (adverse reactions) are very common with antipsychotic treatment. Overall, the safety data from the cariprazine studies demonstrated that it was generally safe and well tolerated in patients with schizophrenia1-3. Most of the side effects that were observed with cariprazine are common with antipsychotic treatment and are expected to be familiar to the practicing clinicians. Additionally, many of the events respond to treatment or are transient. The most frequently reported side effects with cariprazine in the therapeutic dose range (1.5-6 mg) were akathisia (19%) and parkinsonism (17.5%)6. Most events however were mild to moderate in severity6.
Table 1. Side effects6
| Very common (≥1/10) | Common (≥1/100 to <1/10) | Uncommon (≥1/1,000 to <1/100) |
| Akathisia Parkinsonism | Weight increased Decreased appetite Increased appetite Dyslipidaemia Sleep disorders (insomnia) Anxiety Sedation Dizziness Dystonias Other EPS Vision blurred Tachyarrhytmia Hypertension Nausea Constipation Vomiting Hepatic enzymes increased Blood creatine phosphokinase increased Fatigue | Anaemia, Eosinophilia Thyroid stimulating hormone decreased Blood sodium abnormal Blood glucose increased Diabetes mellitus Suicidal behaviour Delirium Depression Libido decreased Libido increased Erectile dysfunction Dysaesthesia Dyskinesias Tardive dyskinesia Eye irritation Intraocular pressure increased Accommodation disorder Visual acuity reduced Vertigo Cardiac conduction disorders Bradyarrhytmia QT prolonged T wave abnormal Hypotension Hiccups Gastrooesophageal reflux Blood bilirubin increased Pruritus Rash Dysuria, Pollakisuria Thirst |
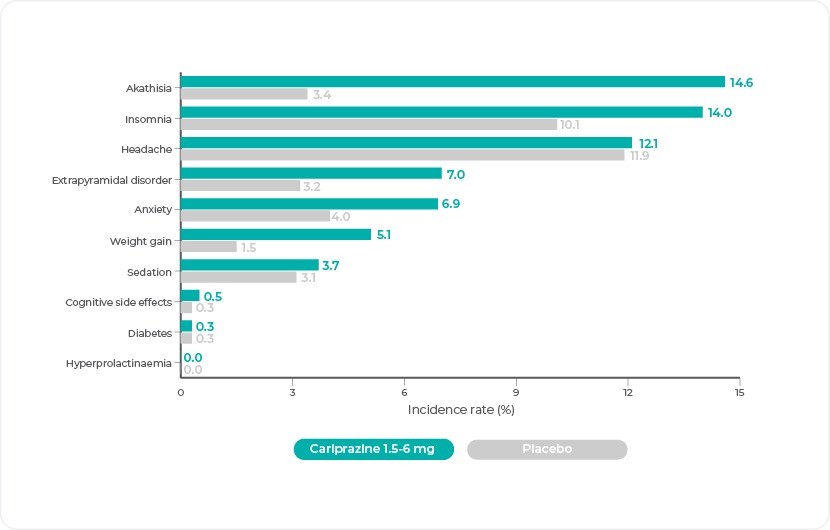
The adverse event profile of cariprazine in the therapeutic 1.5-6 mg/d dose range shows that akathisia, insomnia, and headache occur at relatively high rates, but only the rate of akathisia is substantially higher with cariprazine than with placebo5. Most events of akathisia were considered mild or moderate in severity6.
Reference: Adapted from data in the Reagila Assessment Report EMA. Reagila Assessment Report5 https://www.ema.europa.eu/en/documents/assessment-report/reagila-epar-public-assessment-report_en.pdf.
Highlights of the Safety Profile for Cariprazine
The general safety profile of cariprazine has some benefits compared with other antipsychotics. These include treatment-related changes related to prolactin levels, sexual dysfunction, weight gain and QT prolongation.
Cariprazine does not cause hyperprolactinemia5; no TEAEs related to elevation of prolactin levels were reported in cariprazine-treated patients in the clinical studies5
The incidence of sexual dysfunction was low in patients treated with cariprazine(1.0%)5
Changes in body weight are a known class effect of antipsychotic medications; in the short term studies, there were slightly greater mean increases in body weight in the cariprazine group (1 kg) compared with the placebo group (0.3 kg); in the long term maintenance of effect study, there was no clinically relevant difference in change of body weight from baseline to end of treatment for cariprazine (1.1 kg) and placebo (0.9 kg)6
Cariprazine is metabolically neutral: rates of hyperlipidemia, hyperglycemia, and diabetes mellitus were comparable to placebo5
Frequencies of cognition-related adverse events were similar to placebo and uncommon5 some evidence suggests that cariprazine may improve cognition7
Cariprazine does not cause serious or severe QT prolongation6
Cariprazine causes less sedation than many other antipsychotics, with rates for cariprazine (3.8%) that are similar to placebo (3.1%)5
Throughout the cariprazine development program, 6 deaths occurred due to completed suicide, but none were judged to be related to cariprazine5
References
- Nasrallah, H. A. et al. The safety and tolerability of cariprazine in long-term treatment of schizophrenia: A post hoc pooled analysis. BMC Psychiatry (2017) doi:10.1186/s12888-017-1459-z.
- Earley, W. et al. Safety and tolerability of cariprazine in patients with acute exacerbation of schizophrenia: A pooled analysis of four phase II/III randomized, double-blind, placebo-controlled studies. Int. Clin. Psychopharmacol. 32, 319–28 (2017).
- Németh, G. et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet 389, 1103–1113 (2017).
- Acosta, F. J. Medication adherence in schizophrenia. World J. Psychiatry 2, 74–82 (2012).
- EMA. Reagila Assessment Report. https://www.ema.europa.eu/en/documents/assessment-report/reagila-epar-public-assessment-report_en.pdf.
- Reagila SmPC.
- Fleischhacker, W. et al. The efficacy of cariprazine in negative symptoms of schizophrenia: Post hoc analyses of PANSS individual items and PANSS-derived factors. Eur. Psychiatry 58, 1–9 (2019).
Therapeutic Advances in Psychopharmacology
The prospects of cariprazine in the treatment of schizophrenia.
REAGILA AND METABOLIC PARAMETERSCARIPRAZINE AND METABOLIC PARAMETER…
Individuals with schizophrenia are more likely than members of the general population to be overweight or obese. Moreover, a large number of patients with schizIndividuals with schizophrenia are more likely than members of the general population to be overweight or obese. Moreover, a large number of patients with schiz
more…AKATHISIA…SHOULD WE BE CONCERNED?AKATHISIA…SHOULD WE BE CONCERNED?
Akathisia is a common side effect of antipsychotic treatment. It is usually considered a movement or extrapyramidal disorder, with motor signs and sensory distuAkathisia is a common side effect of antipsychotic treatment. It is usually considered a movement or extrapyramidal disorder, with motor signs and sensory distu
more…