REAGILA Controls Negative Symptoms in Schizophrenia
- Cariprazine is more effective in controlling negative symptoms of schizophrenia than risperidone3
- The overall efficacy of cariprazine in negative symptoms can be attributed to improvement across individual symptoms4
- Cariprazine has the potential to change clinical practise by providing a treatment option for patient with predominant negative symptoms of schizophrenia3
In this section
The Trouble With Primary Negative Symptoms
Negative symptoms of schizophrenia can occur as primary symptoms that are part of the underlying pathophysiology of schizophrenia or as secondary symptoms that are associated with or caused by other factors, such as positive symptoms, affective symptoms, or medication side effects5. Secondary negative symptoms are often responsive to treatment of the underlying cause—for example, negative symptoms that are associated with positive symptoms may well improve as a function of positive symptom improvement2. On the other hand, primary negative symptoms have generally been considered unresponsive to treatment with antipsychotics or other agents3.
Negative Symptom Efficacy With Cariprazine
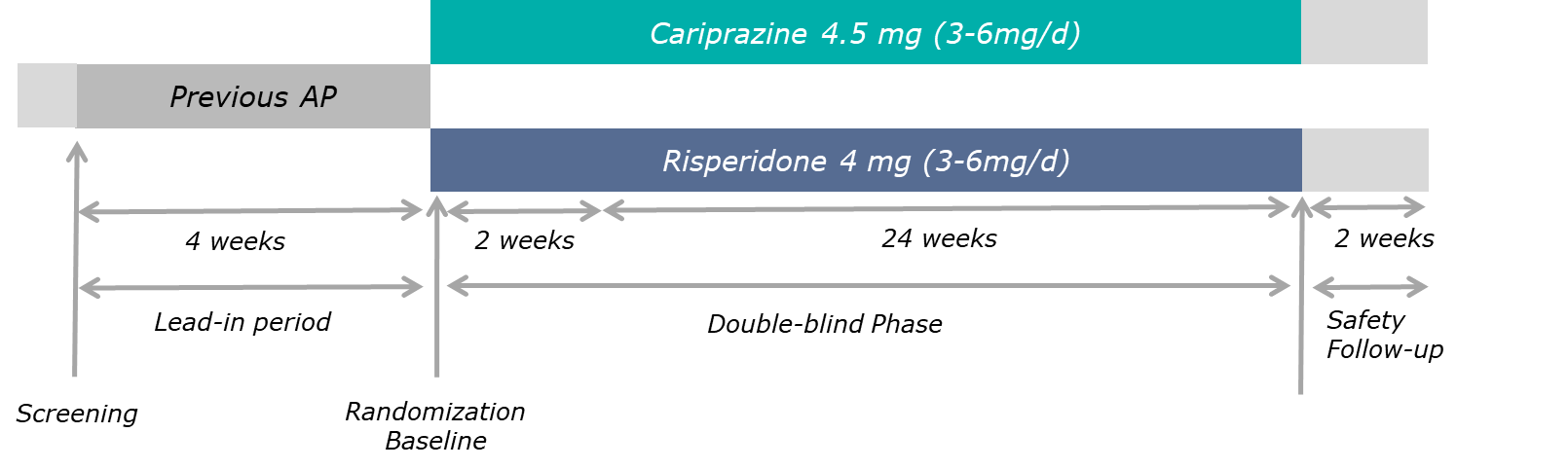
Cariprazine Versus Risperidone for the Treatment of Persistent and Predominant Negative Symptoms3,25 (Eudra CT number: 2012-005485-36)
Persistent and predominant negative symptoms have been evaluated in one Phase 3b, multinational, all European, multicenter, randomized, double-blind, active-referenced, parallel-group, fixed/flexible-dose study in stable adult patients with predominant negative symptoms of schizophrenia. The study was prospectively designed to be in alignment with EMA and FDA guidelines for studies evaluating negative symptoms6,7.
Risperidone, an atypical antipsychotic with proven efficacy in schizophrenia, was the active control; 4 mg/d was selected as the target dose for risperidone to correspond to the usual daily dose for patients with stable schizophrenia and to minimize the occurrence of extrapyramidal symptoms8. Risperidone was chosen among the second generation antipsychotics based on the following reasoning: According to a large meta-analysis, four antipsychotics (olanzapine, risperidone, amisulpride and clozapine) are more efficacious than first generation antipsychotics in treating negative symptoms, although none proved to be superior over the other three9. Therefore choosing any of these medications as an active comparator for a negative symptom study could have been a reasonable option. The final choice was put on risperidone, because clozapine is not used as a first line treatment10 and olanzapine could have potentially unblinded the study due to its side effect profile (mainly weight gain)11. Amisulpride is used in two different dose ranges, depending on whether positive (target dose is 400- 1200 mg/day) or negative symptoms (50-300 mg/day) are to be controlled12. Since the aim of the study was to keep positive symptoms stable and to improve negative symptoms, the choice of the amisulpride dose would have been a difficult one to make; especially since this was a fixed dose study where in case of an impending relapse the dose of amisulpride could not have been adjusted. Additional aspects were that amisulpride is not available in various European countries and that it has a different side effect profile from that of cariprazine (higher incidence of sedation). Considering all these aspects, the decision was made to use risperidone as the best possible comparator, knowing there is no ideal choice.
To ensure that improvements in negative symptoms were not secondary to improvements in other symptom domains, patients with positive symptoms, moderate or severe depressive symptoms, or clinically relevant parkinsonism were excluded. Additional inclusion and exclusion criteria were also applied to ensure that change in negative symptoms was genuine and not the result of changes in other symptoms that can affect negative symptoms3.
There were 3 study periods: a 4-week prospective lead-in period, a 2-part, 26-week randomized (1:1) double-blind treatment period (2-week cross-titration where randomized treatment was up-titrated to the target dose and the previous antipsychotic was withdrawn; and 24-week maintenance of target dose), and a 2-week safety follow-up period. For double-blind treatment, patients received the target doses of cariprazine 4.5 mg/day (n=227) or risperidone 4.0 mg/day (n=229)3,25.
Study Design
Reference: Németh, G. et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet 389, 1103–1113 (2017)3 (Graphic elaboration from text data)
The primary outcome measure was change from baseline to week 26 in PANSS-factor score for negative symptoms (PANSS-FSNS)13; a higher score indicates worse severity. This is a modified score as compared to the negative subscale of the PANSS (excluding 2 cognitive items of the negative subscale and including 2 items from the general psychopathology that are relevant for negative symptoms)3.
| PANSS Negative | Subscale | Factor |
| N1: Blunted affect | x | x |
| N2: Emotional withdrawal | x | x |
| N3: Poor rapport | x | x |
| N4: Passive/apathetic social withdrawal | x | x |
| N5: Difficulty in abstract thinking | x | |
| N6: Lack of spontaneity and flow of conversation | x | x |
| N7: Stereotyped thinking | x | |
| G7: Motor retardation | x | |
| G16: Active social avoidance | x |
References: Marder, S. R., Davis, J. M. & Chouinard, G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: Combined results of the North American trials. J. Clin. Psychiatry 58, 538–546 (1997)13; Kay, S. R., Fiszbein, A. & Opler, L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 13, 261–276 (1987)14 (Graphic elaboration from text data)
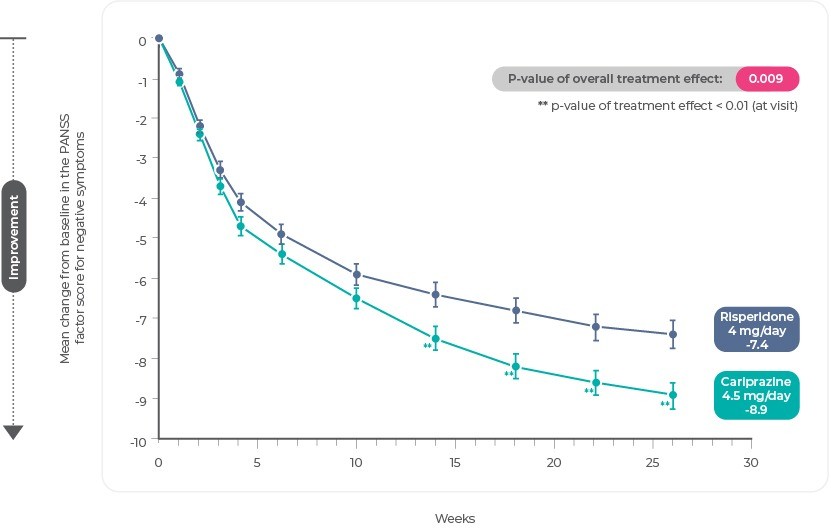
The least squares mean change from baseline to week 26 in PANSS-FSNS was statistically significant in favor of cariprazine (-8.90) versus risperidone (-7.44); a statistically significant separation between treatments began at week 14 and persisted until the end of the study25. The least squares mean difference (LSMD) was -1.46 (95% CI, -2.39,-0.53; P=.0022; effect size=0.31)3.
PANSS-FSNS Change From Baseline
Reference: Adapted from Németh, G. et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet 389, 1103–1113 (2017)3.
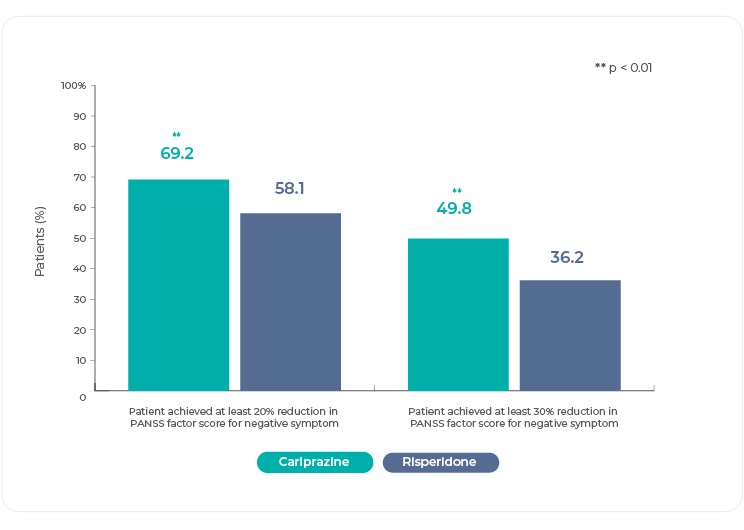
Response (decrease ≥20% in PANSS-FSNS by week 26) was achieved by significantly more cariprazine-treated patients (69%) than risperidone-treated patients (58%) (odds ratio=2.08; P=.0022); the number needed to treat (NNT) was 925. In post-hoc analyses, response was also assessed with a more stringent response criterion (decrease ≥30% in PANSS-FSNS by week 26). Again, the rate of response was significantly greater for cariprazine- (50%) than risperidone-treated (36%) patients (P=.0033), with an NNT of 825. These increased PANSS-FSNS response rates for cariprazine compared with risperidone supported the clinical significance of the cariprazine treatment effect in this study3,25.
Reference: Németh, G. et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet 389, 1103–1113 (2017)3 (Graphic elaboration from text data)
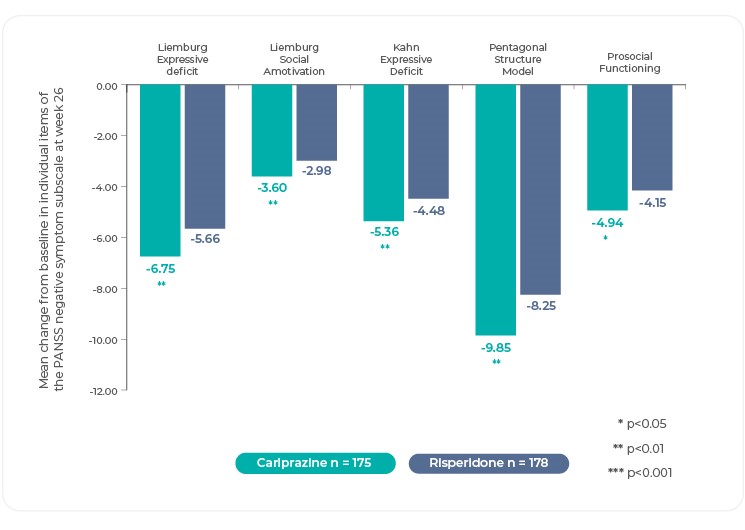
Individual Negative Symptom Efficacy With Cariprazine
The heterogeneity of negative symptoms suggests that there may be different underlying pathophysiological mechanisms and psychopathological outcomes15,16. Understanding how cariprazine performs across the range of negative symptoms illuminates its overall efficacy25.
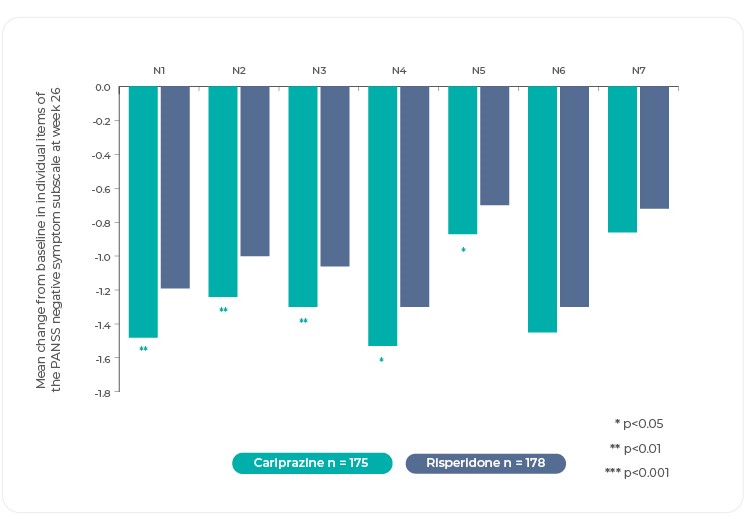
Changes in individual negative symptom items as measured by the PANSS, and PANSS based factors were investigated in post hoc analyses4. The difference in mean change from baseline was statistically significant in favor of cariprazine versus risperidone on most items of the PANSS Negative Symptom subscale as well as on the overall subscale itself25. Of note, differences in mean change on several of the items that evaluate the key negative symptom constructs were statistically significant in favor of cariprazine: blunted affect, avolition (measured by N2, emotional withdrawal), asociality (measured by N3, poor rapport and N4, passive/apathetic social withdrawal)4,25. Although there is no item for anhedonia on the Negative Symptoms subscale, improvement for cariprazine can be inferred from greater change on items like N2 and N4, which suggest that patients are experiencing a lack of interest and less social involvement25. Interestingly, no significant between-group difference was seen on stereotyped thinking, but this item is no longer considered part of the negative symptom domain according to negative symptom factor analyses4.
PANSS Negative Symptom Subscale
Reference: Adapted from Fleischhacker, W. et al. The efficacy of cariprazine in negative symptoms of schizophrenia: Post hoc analyses of PANSS individual items and PANSS-derived factors. Eur. Psychiatry 58, 1–9 (2019)4.
PANSS-Based Factors
The structure of negative symptoms is multidimensional, with evidence generally supporting models that consist of 2 to 5 factors17. The most stable and replicated models have 2 factors clustering into the potentially related but distinct subdomains: diminished expression (blunted affect and alogia) and avolition/apathy (avolition, asociality, anhedonia). Data from the negative symptom trial evaluating cariprazine versus risperidone were evaluated in several PANSS-derived factor models13,18-23. On all factor models, statistically significant improvement was demonstrated for cariprazine versus risperidone4,25.
Reference: Adapted from Fleischhacker, W. et al. The efficacy of cariprazine in negative symptoms of schizophrenia: Post hoc analyses of PANSS individual items and PANSS-derived factors. Eur. Psychiatry 58, 1–9 (2019)4.
Finally, an Effective Treatment for Negative Symptoms
After decades of research and investigation, one monotherapy atypical antipsychotic has proven its efficacy against another representative, atypical antipsychotic in the treatment of negative symptoms of schizophrenia3. Cariprazine is effective against negative symptoms of schizophrenia—a common and debilitating aspect of a chronic and severe neuropsychiatric disorder25. Until now, negative symptoms have been resistant to treatment, representing an important unmet medical need for patients with schizophrenia. Since not all patients are responsive to any one medication, more treatment options for negative symptoms are still needed, but patients who have shown improvement in positive symptoms but continue to have disabling negative symptoms while on an antipsychotic other than cariprazine, might benefit from treatment with cariprazine3,25. That is why in a recent publication cariprazine was suggested as the first step in the algorithm of negative symptom treatment24.
References
- Krause, M. et al. Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: a systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 268, 625–639 (2018).
- Leucht, S. et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: Systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am. J. Psychiatry 174, 927–942 (2017).
- Németh, G. et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet 389, 1103–1113 (2017).
- Fleischhacker, W. et al. The efficacy of cariprazine in negative symptoms of schizophrenia: Post hoc analyses of PANSS individual items and PANSS-derived factors. Eur. Psychiatry 58, 1–9 (2019).
- Buchanan, R. W. Persistent Negative Symptoms in Schizophrenia: An Overview. Schizophr. Bull. 33, 1013–1022 (2007).
- Laughren, T. & Levin, R. Food and drug administration commentary on methodological issues in negative symptom trials. Schizophr. Bull. 37, 255–256 (2011).
- EMA. Guideline on clinical investigation of medicinal products, including depot preparation in the treatment of schizophrenia. (2012).
- Risperidone smpc. http://mri.cts-mrp.eu/download/NL_H_1078_002_FinalPI.pdf.
- Leucht, S. et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 373, 31–41 (2009).
- Clozapine smpc. https://www.medicines.org.uk/emc/product/4411/smpc.
- Olanzapine smpc. https://www.medicines.org.uk/emc/product/4091/smpc.
- Amisulpride smpc. https://www.medicines.org.uk/emc/product/4091/smpc.
- Marder, S. R., Davis, J. M. & Chouinard, G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: Combined results of the North American trials. J. Clin. Psychiatry 58, 538–546 (1997).
- Kay, S. R., Fiszbein, A. & Opler, L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 13, 261–276 (1987).
- Bucci, P. & Galderisi, S. Categorizing and assessing negative symptoms. Curr. Opin. Psychiatry 30, 201–208 (2017).
- Galderisi, S., Mucci, A., Buchanan, R. W. & Arango, C. Negative symptoms of schizophrenia: new developments and unanswered research questions. The Lancet Psychiatry 5, 664–677 (2018).
- Blanchard, J. J. & Cohen, A. S. The structure of negative symptoms within schizophrenia: Implications for assessment. Schizophr. Bull. 32, 238–245 (2006).
- Liemburg, E. et al. Two subdomains of negative symptoms in psychotic disorders: Established and confirmed in two large cohorts. J. Psychiatr. Res. 47, 718–725 (2013).
- Khan, A. et al. Negative symptom dimensions of the positive and negative syndrome scale across geographical regions: Implications for social, linguistic, and cultural consistency. Innov. Clin. Neurosci. 14, 30–40 (2017).
- White, L. et al. Empirical assessment of the factorial structure of clinical symptoms in schizophrenia: A multisite, multimodel evaluation of the factorial structure of the positive and negative syndrome scale. Psychopathology 30, 263–274 (1997).
- Fervaha, G., Foussias, G., Agid, O. & Remington, G. Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta Psychiatr. Scand. 130, 290–299 (2014).
- Purnine, D. M., Carey, K. B., Maisto, S. A. & Carey, M. P. Assessing positive and negative symptoms in outpatients with schizophrenia and mood disorders. J. Nerv. Ment. Dis. 188, 653–661 (2000).
- Meltzer, H. Y. et al. Lurasidone in the treatment of schizophrenia: A randomized, double-blind, placebo- and olanzapine-controlled study. Am. J. Psychiatry 168, 957–967 (2011).
- Cerveri, G., Gesi, C. & Mencacci, C. Pharmacological treatment of negative symptoms in schizophrenia: update and proposal of a clinical algorithm. Neuropsychiatr. Dis. Treat. 15, 1525–1535 (2019).
- Reagila SmPC.
European Psychiatry
The efficacy of cariprazine in negative symptoms of schizophrenia: Post hoc analyses of PANSS individual items and PANSS-derived factors.
REAGILA AND DAILY FUNCTIONINGOUR PRODUCT AND DAILY FUNCTIONING
Negative symptom improvement must be accompanied by improved patient functioning in order for change to be considered clinically relevant in patients with schizNegative symptom improvement must be accompanied by improved patient functioning in order for change to be considered clinically relevant in patients with schiz
more…WHAT’S THE PSEUDOSPECIFICITY ISSUE?WHAT’S THE PSEUDOSPECIFICITY ISSUE?
Although cariprazine is the first and only antipsychotic to demonstrate efficacy against another antipsychotic in a large-scale trial specially designed to evalAlthough cariprazine is the first and only antipsychotic to demonstrate efficacy against another antipsychotic in a large-scale trial specially designed to eval
more…